Dynamic images show rhomboid protease in action
Rhomboid proteases are clinically relevant membrane proteins that play a key role in various diseases. Using solid-state NMR spectroscopy, researchers from Berlin’s Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) have now been able to watch rhomboid proteases in a native lipid environment at work. The obtained dynamic images will be useful for the development of new medication for diseases such as Parkinson’s and malaria.
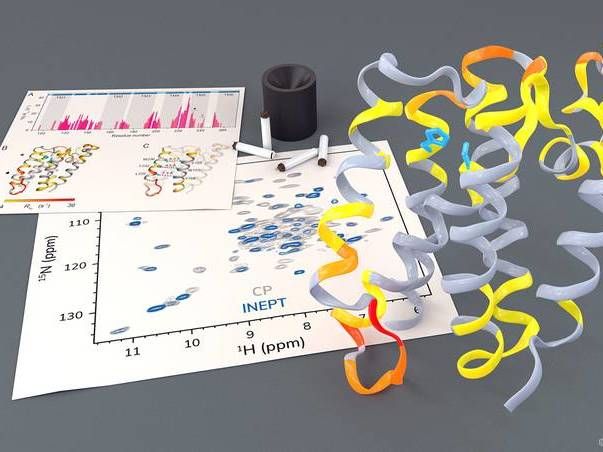
Investigation of the rhomboid protease GlpG by solid-state NMR.
Barth van Rossum, FMP
Tens of thousands of proteins are at work in our cells, around the clock. Some of these industrious workers sit in the cell membrane, among them the family of rhomboid proteases. Given that these intramembrane proteases are involved in many biological processes, and also play a key role in diseases such as Parkinson’s, diabetes, cancer and malaria, they are highly clinically relevant.
Previously, it had been possible to view rhomboid proteases using X-ray crystallography. However, this method was only able to provide static images from proteins in an artificial environment. Therefore it remained of great interest to see what happens in the cell membrane where the proteins go about their main task, which is cleaving other membrane proteins, triggering a signaling cascade.
The long-suspected gate that opens does in fact exist
The research group led by Professor Adam Lange from the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) has now been able to investigate this highly complex process, for the first time using solid-state NMR spectroscopy in a native-like environment. The researchers were able to observe how certain parts of the protease move. They also noticed that, in order to cleave other proteins, a gate opens briefly to let these substrate proteins enter the active center of the protease.
Findings relevant for pharmacological interference
The project, undertaken within the UniSysCat Cluster of Excellence, sets a base for an even better characterization of rhomboid proteases. What is more: The knowledge gained will be useful for researchers to investigate how they can pharmacologically influence the clinically relevant membrane proteins. Also Lange and his team now want to search for substances to inhibit errant rhomboid proteases.
Original publication
Chaowei Shi*, Carl Öster*, Claudia Bohg, Longmei Li, Sascha Lange, Veniamin Chevelkov, Adam Lange; "Structure and Dynamics of the Rhomboid Protease GlpG in Liposomes Studied by Solid-State NMR"; Journal of the American Chemical Society; October 2019; *equally contributing first authors.
Original publication
Chaowei Shi*, Carl Öster*, Claudia Bohg, Longmei Li, Sascha Lange, Veniamin Chevelkov, Adam Lange; "Structure and Dynamics of the Rhomboid Protease GlpG in Liposomes Studied by Solid-State NMR"; Journal of the American Chemical Society; October 2019; *equally contributing first authors.
Organizations
Other news from the department science
These products might interest you

Kjel- / Dist Line by Büchi
Kjel- and Dist Line - steam distillation and Kjeldahl applications
Maximum accuracy and performance for your steam distillation and Kjeldahl applications

AZURA Purifier + LH 2.1 by KNAUER
Preparative Liquid Chromatography - New platform for more throughput
Save time and improve reproducibility during purification

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.