64 human genomes as new reference for global genetic diversity
The new reference data provide an important basis: The aim is to estimate the individual risk of developing certain diseases such as cancer
Exactly 20 years after the successful completion of the "Human Genome Project", an international group of researchers, the Human Genome Structural Variation Consortium (HGSVC), has now sequenced 64 human genomes at high resolution. This reference data includes individuals from around the world, better capturing the genetic diversity of the human species. Among other applications, the work enables population-specific studies on genetic predispositions to human diseases as well as the discovery of more complex forms of genetic variation, as the 65 authors report in the current issue of the scientific journal Science.
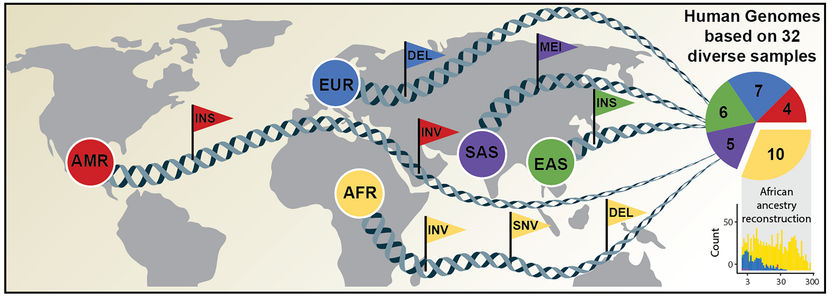
Comprehensive discovery of genetic variation based on analysis of human genomes of diverse ancestry.
University of Washington / David Porubsky
In 2001, the “International Human Genome Sequencing Consortium” announced the first draft of the human genome reference sequence. The Human Genome Project, as it was called, had taken more than eleven years of work and involved more than 1000 scientists from 40 countries. This reference, however, did not represent a single individual but instead is a composite of humans that could not accurately capture the complexity of human genetic variation.
Building on this, scientists have carried out many sequencing projects over the last 20 years to identify and catalog genetic differences between an individual and the reference genome. Those differences usually focused on small single base changes and missed larger genetic alterations. Current technologies now are beginning to detect and characterize larger differences – called structural variants – such as insertions of several hundred letters. Structural variants are more likely than smaller genetic differences to interfere with gene function.
An international research team has now published an article in Science announcing a new, considerably more comprehensive reference dataset obtained using a combination of advanced sequencing and mapping technologies. The new reference dataset reflects 64 assembled human genomes, representing 25 different human populations from across the globe. Importantly, each of the genomes was assembled without guidance from the first human genome and as a result better captures genetic differences from different human populations. The study was led by scientists from the European Molecular Biology Laboratory Heidelberg (EMBL), the Heinrich Heine University Düsseldorf (HHU), The Jackson Laboratory for Genomic Medicine in Farmington, Conn. (JAX), and the University of Washington in Seattle (UW).
“With these new reference data, genetic differences can be studied with unprecedented accuracy against the background of global genetic variation, which facilitates the biomedical evaluation of genetic variants carried by an individual," emphasizes the co-first author of the study, Dr. Peter Ebert from the Institute of Medical Biometry and Bioinformatics at HHU. The distribution of genetic variants can differ substantially between population groups as a result of spontaneous and continuously occurring changes in the genetic material. If such a mutation is passed on over many generations, it can become a genetic variant specific to that population.
The new reference data provide an important basis for including the full spectrum of genetic variants in so-called genome-wide association studies. The aim is to estimate the individual risk of developing certain diseases such as cancer and to understand the underlying molecular mechanisms. This, in turn, can be used as a basis for more targeted therapies and preventative medicine.
This work might enable further applications in precision medicine. Drug efficacy, for example, can vary between individuals based on their genomes. The new reference data now represent the full range of different genetic variant types and incorporates human genomes of great diversity. Therefore, this new resource might contribute to developing novel approaches in personalized medicine, where the selection of therapies is tailored to a patient’s individual genetic background.
This study builds on a new method published by these researchers last year in Nature Biotechnology [DOI 10.1038/s41587-020-0719-5] to accurately reconstruct the two components of a person's genome – one inherited from a person’s father, one from a person’s mother. When assembling a person’s genome, this method eliminates the potential biases that could result from comparisons with an imperfect reference genome.