New test to make gene therapy safer
SAGA detects tumour potential of viral vectors for blood stem cells
When the causes of serious diseases are based on a defective gene, medicine relies on gene therapy. In this strategy, defect-free genes are introduced into the body with the help of certain viruses. In this way, individual forms of congenital blindness, hereditary muscular atrophy or certain diseases of the blood and immune system can already be treated today. However, the viral vectors used as gene shuttles work in different ways. Some simply deliver the intact gene into the cell. The others belong to the retroviruses and incorporate the functional copy firmly into the genome. This has the advantage that the healthy copy of the gene is passed on to all descendants of the treated stem cell and the resulting cells during cell division. However, there is a risk: the retroviral gene shuttles can sometimes switch on cancer genes and thus trigger leukaemia. Now, an international research team led by Dr. Michael Rothe from the Institute of Experimental Haematology at the Hannover Medical School (MHH) has developed a safety test that can accurately predict the potential cancer risk of retroviral vectors. The research results have been published in the journal Molecular Therapy.
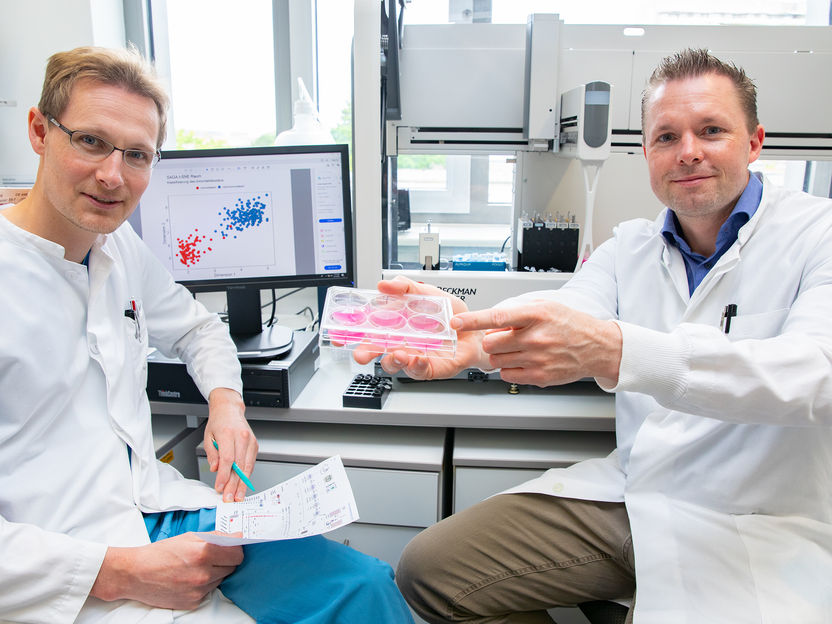
Dr. Michael Rothe (right, with a cell culture cultivation dish) and Dr. Dr. Adrian Schwarzer in front of a pipetting robot for testing gene therapy vectors.
Copyright: Karin Kaiser / MHH
Promising therapy for monogenetic diseases
The scientists focused on the so-called haematopoietic stem cells of the blood. These blood stem cells are formed in the bone marrow, among other places, and can produce red blood cells, blood platelets and also the various white blood cells that are important for the immune system. "Blood stem cell gene therapy with retroviral vectors is a promising therapy for blood diseases that are only caused by a single defective gene," says Dr. Dr. Adrian Schwarzer, medical doctor at the Department of Haematology, Haemostaseology, Oncology and Stem Cell Transplantation and first author of the publication. In some clinical studies, however, individual patients developed leukaemia after they had been transplanted with the genetically modified blood stem cells. "This happens when the vector inserts itself near cancer genes and activates them," explains Dr. Rothe.
SAGA reliably filters out genotoxic vectors
To prevent this side effect, the vectors are usually tested in the mouse model before they are used on patients. "This is lengthy, and unfortunately not always very conclusive," the scientist emphasises. The IVIM (In Vitro Immortalisation) test developed at the Institute of Professor Dr Axel Schambach several years ago and approved in the USA, Canada, Australia and Europe already detects problematic vectors in cell culture. But the new safety test can do even more. The scientists discovered that genotoxic vectors specifically alter the conversion of the genetic information of some genes and thus leave a typical trace. The scientists use artificial intelligence to recognise this gene expression signature from eleven genes when blood stem cells are treated with the gene therapy vectors in cell culture. "SAGA has filtered out all known cancer-causing vectors," says Dr Rothe. Through machine learning, he says, the system is also able to give a quick and reliable risk assessment for newly developed vectors for stem cell gene therapy. The results pave the way for safer gene therapy studies and should exclude leukaemia as a possible dangerous side effect in the future.
Original publication
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.