Infrared in the heel
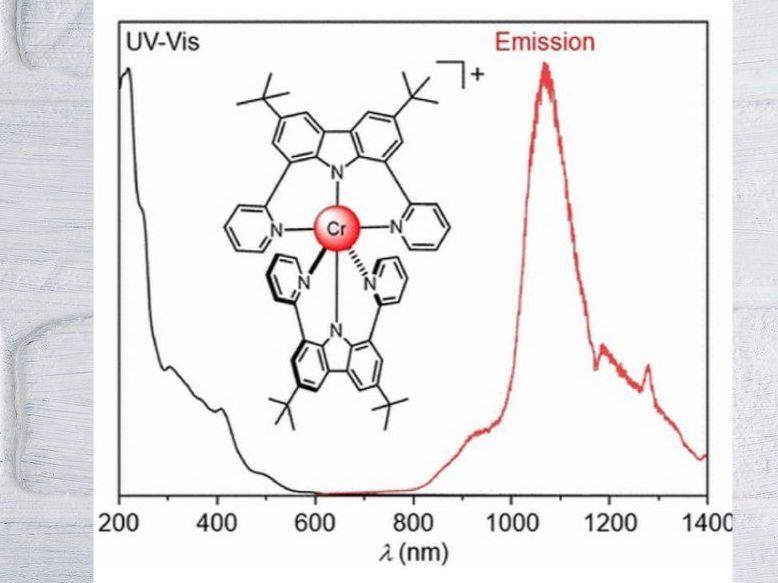
A chromium complex as NIR-II emitter
Substances that emit light in the near infrared (NIR) range are needed for a wide variety of applications, from fibre-optic telecommunications to imaging techniques for biomedicine. A Swiss research team has now for the first time developed a chromium complex that emits light in the coveted longer-wavelength NIR-II range. In the journal Angewandte Chemie, the team presents the underlying concept: A drastic change in the electronic structure at the chromium using specially adapted ligands encompassing the chromium.
Many of the NIR light-emitting materials are based on expensive or rare metal complexes. Less expensive alternatives emitting in the NIR-I region between 700 and 950 nm have now been developed, while NIR-II emitting complexes of base metals remain extremely rare. luminescence in the NIR-II region (1000 to 1700 nm) is particularly advantageous for in vivo imaging because this light penetrates very deeply into tissue.
The luminescence of complexes is based on an excitation of electrons, e.g. by absorption of light. When the excited electron falls back to the ground state, part of the energy is emitted as radiation. Its wavelength depends on the energetic differences between the electronic states. In complexes, these are determined to a large extent by the type and arrangement of the ligands bound to the metal.
While in typical chemical (covalent) bonds both partners contribute one electron each to an electron pair bond, in many complexes the bonding electrons both originate from the ligand. However, the transitions are smooth: metal-ligand bonds can be partially covalent in character (nephelauxetic effect). As a consequence, the energy of certain excited states is reduced and the emitted radiation is longer wavelength. This is observed, for example, for polypyridine ligands, which shift the intrinsically ruby-red emission of trivalent chromium (CrIII) in complexes up to the NIR-I.
To further increase the covalency of the metal-ligand bond and thus the wavelength, Narayan Sinha in the team led by Claude Piguet and Oliver S. Wenger of the Universities of Basel and Geneva switched from classical polypyridine ligands to a new tailored charged tridentate chelate ligand. Chelate derives from the Greek word for crab scissors; tridentate means that the ligand has three binding sites with which it "pincers" the central metal atom.
In the resulting complex, the CrIII ion is embraced on all sides by two of the new tridentate chelating ligands in an octahedral shape. The result is a drastically altered, unusual electronic structure with a high electron density at CrIII. Charge transfers occur from the ligands to the metal in the axial direction, but from the metal to the ligands in the equatorial plane of the octahedron. Presumably, these combined "pushing" and "pulling" interactions have a strong influence on the spectroscopically relevant electrons of CrIII - the key to the NIR-II emission of the new complex.
Infrared in the heel
Wiley-VCH
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.






















































