Hepatitis: 3D structure determination of the ‘gateway’ to the liver
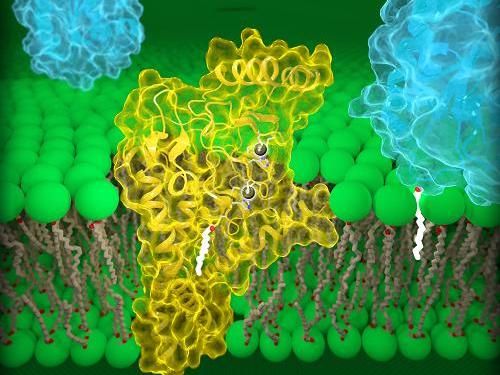
Though an essential gateway to the liver, NTCP had not been well described until now. Na+-taurocholate co-transporting polypeptide (NTCP) is a protein located exclusively in the membrane of liver cells that enables recycling of bile acid molecules. It is also the cellular receptor of human hepatitis B and D viruses (HBV/HDV). A better understanding of NTCP could enable the development of treatments specifically designed for the liver, and to fight HBV and HDV infection.
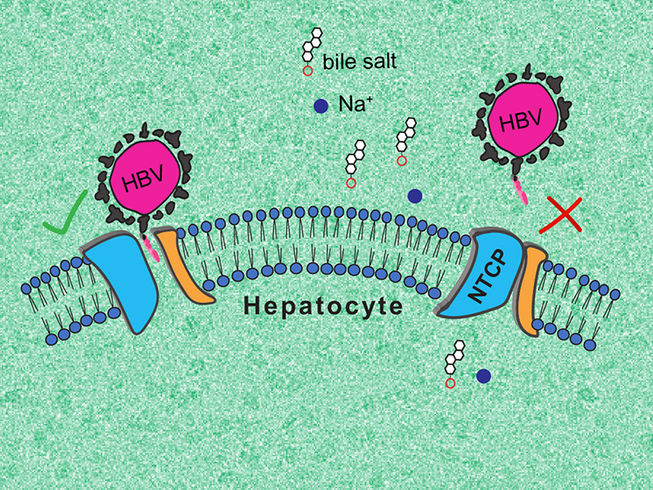
Illustration of the two 3D conformations adopted by NTCPs. Left: ‘open’ conformation to which HBV and HDV can bind. Right: ‘closed’ conformation that prevents recognition by the viruses.
© Kapil Goutam/Nicolas Reyes/CNRS
NTCP is a difficult protein to study. It weighs only 38 kilodaltons (kDa), whereas cryo-electron microscopy, the technology used to study this type of molecule, only works for molecules weighing more than 50 kDA. The challenge was therefore to “enlarge” and stabilise it.
To do this, teams from French and Belgian laboratories developed and tested a collection of antibody fragments targeting NTCP. The 3D structures of the resulting complexes were determined using cryo-electron microscopy, and different antibody fragments stabilised and revealed several forms of NTCP.
The research team was able to describe two essential NTCP conformations: one in which the protein opens a large membrane pore to bile salts, to which HBV and HDV can bind, and a second, ‘closed’ conformation, that prevents recognition by the viruses.
The first, ‘open’ conformation is very surprising, as no other known molecular transporter forms such a ‘wide open’ pore. In turn, the second conformation could help finding antiviral molecules that prevent HBV and HDV infection. The research team intends to continue its work to fully elucidate the functioning of NTCP.
Most read news
Organizations

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.