Imaging the dynamic cellular zoo made easier
This work will expand the ability to simultaneously monitor many processes in cells
Imagine the difficulty of visually keeping track of five people scattered throughout a stadium. Researchers perform far more amazing feats by simultaneously tracking many different cellular factors, but they need an expanded fluorescence toolkit to advance current capabilities.
Symbolic image
Computer-generated image
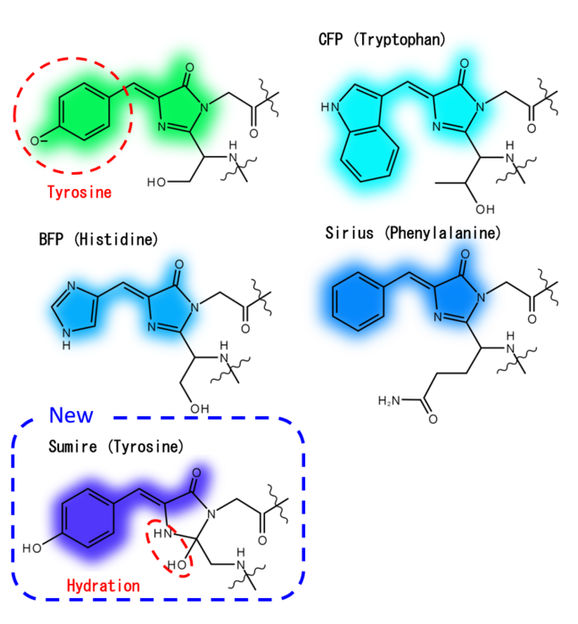
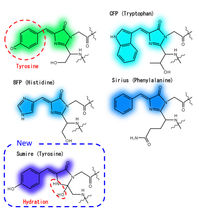
Chromophore structure of short wavelength side avGFP mutants
Kazunori Sugiura
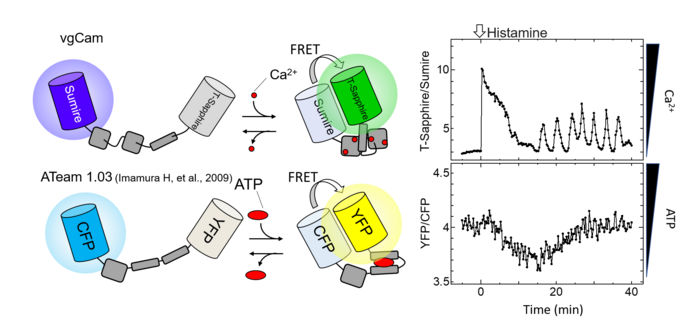
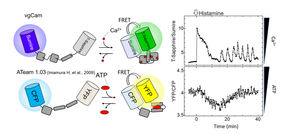
Schematic of FRET-type probe (left) as well as calcium and ATP concentration changes in the same cell (right). Addition of histamine at 0 min.
Kazunori Sugiura
Now, in a study recently published in Communications Biology, researchers from SANKEN (The Institute of Scientific and Industrial Research) at Osaka University have genetically modified a protein to exhibit the shortest fluorescence emission wavelength currently available.
Fluorescence is a common means of microscopically visualizing the inner workings of cells. For example, a biomolecule of interest can be genetically appended with a fluorescent protein (i.e., a fluorophore) that emits a specific color (i.e., wavelength) of light. By appending different types of biomolecules with different fluorophores, each emitting a different wavelength of light, one can identify and track all of these different biomolecules at once. Expanding the range of possible emitted wavelengths can expand the number of biomolecules that can be simultaneously tracked. This is the problem that the researchers sought to address.
"The short-emission wavelength limit of fluorescent proteins has stayed the same for the past 10 years," explains Kazunori Sugiura, lead author. "This is because previous researchers have usually focused on making minor changes to one of the amino acids of green fluorescent protein mutants."
The Osaka University researchers instead focused on optimizing the interactions between the fluorescence center (i.e., the chromophore) and the surrounding water molecules and amino acids. By hindering ionization and stabilizing hydration of the chromophore, the resulting fluorophore—termed Sumire—exhibited several noteworthy fluorescence properties: (1) emission at 414 nanometers, a new record; (2) brightness nearly four times the state-of-the-art; and (3) stable emission from pH 5.5–9.0, which encompasses most of the pH range seen in most cells.
"We also achieved fluorescence resonance energy transfer, a common biomolecular imaging technique, between Sumire and common commercial protein fluorophores," says Takeharu Nagai, senior author. "This further illustrates the compatibility of Sumire with modern multi-parameter analysis."
This work succeeded in using genetic engineering to expand the cellular imaging toolkit, by modifying the chromophore of a fluorescent protein in a manner that had not yet been considered. The Osaka University researchers' approach will be useful for further expanding the range of attainable fluorescence wavelengths from engineered proteins, which will help researchers uncover biological principles that are important in normal health and disease.