Shedding New Light on Viruses
Analysis of viruses with a combination of mass spectrometry and X-ray laser
Professor Dr. Charlotte Uetrecht from the University of Siegen is studying the structures of proteins and protein complexes in coronaviruses and noroviruses, and how they change over the viral lifecycle. She is developing a new method to obtain as much information as possible about states of these viral building blocks which only exist briefly. The resulting in-depth knowledge of the material conversions taking place in the viruses may lead to a new method of attack for antiviral drugs.

Prof. Dr. Charlotte Uetrecht
Sascha Hüttenhain
Before the COVID-19 pandemic, Prof. Dr. Charlotte Uetrecht sometimes had to explain at length why it‘s important to study the structure of coronaviruses. Today, explanations are necessary above all for her second research subject, noroviruses. »Noroviruses are highly contagious and cause diarrhea with vomiting. The sickness, often called stomach flu, is a risk above all for people with a weak immune system,« says the professor. She also points out the economic costs of sick leave caused by what many people think of as harmless infections.
To take a closer look at the structure of viruses, Uetrecht uses a state-of-the-art type of mass spectrometry termed native mass spectrometry. The established form of mass spectrometry has been around for about 100 years. It involves ionizing molecules of substances into electrically charged particles, in other words converting them into ions. Then the analysis device separates the ions according to their mass-to-charge ratio and registers them.
For a long time, mass spectrometry was not suitable for analyzing proteins and protein complexes (assemblies of proteins) which are important in medicine and biology. It wasn‘t possible to ionize these very large, heavy molecules. The other problem was that the analysis substances were dissolved in liquid solvents, but this destroys the structure of proteins. In the late 1990s, scientists further developed the technology and ionization method so that it became possible to examine undamaged proteins and protein complexes in their original biological state. This was the start of native mass spectrometry.
Insights into viral replication
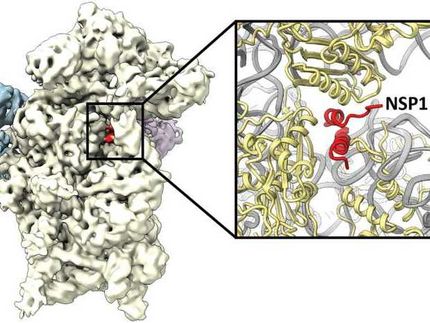
Using this method, Uetrecht and her team have explained details of processes taking place during formation of the replication and transcription complex (RTC) of corona-viruses. This complex is part of a viral mechanism that is responsible for the replication of the genome, or the RNA of the virus. »A possible approach for antiviral medicines is attacking the processes the RTC is involved in,« says Uetrecht. Medicines like this could be targeted to suppress the formation of viral offspring.
The study focused on the proteins nsp7 and nsp8 and their temporary aggregation into complexes. These proteins regulate various enzymes in the RTC, including the RNA polymerase, which plays a role in the formation of the RNA strand from its basic building blocks. The team of researchers headed by Uetrecht analyzed the nsp7+8 complexes of seven different coronavirus strains. These strains included the pathogens of the respiratory diseases SARS and MERS, the cause of the COVID-19 pandemic, and the pathogens of the cat disease FIP, which is often fatal for these animals.
The researchers discovered that there are differences between the nsp7+8 protein complexes of the different virus strains. Some of them, e.g. for SARS-CoV-2, consist of two nsp7 and two nsp8 units, while the complex for the FIP virus consists of two nsp7 and only one nsp8 protein. A third virus group forms complexes of both four and three units. »Based on our findings, we proposed a model that explains the composition of the different nsp7+8 complexes. The findings help us to better understand the exact function and role of nsp7 and nsp8 in the replication and transcription complex. This also allows us to better understand the replication of coronaviruses,« says Uetrecht.
Virus shells as transporters
In the case of noroviruses, Uetrecht is interested above all in the structure of the protein shell. This outer shell is key to enabling the virus to connect with and penetrate human cells, or, in other words, to cause infection. Another significant factor is that the human immune system recognizes viruses by their surface proteins. Therefore, knowledge of the shell structure is also important for vaccine research. So far, we don‘t have any commercial vaccines against noroviruses.
As it‘s extremely difficult to cultivate intact human noroviruses in human cell cultures, Uetrecht and her team use insect cells. They allow these cells to produce the VP1 protein which then self-assembles empty virus particles. The structures created are particles similar to virus-like particles, and ideal for studying the properties of the shells. Uetrecht and her team have shown that the shells of various norovirus strains display different degrees of stability.
»Virus-like particles are technologically interesting as vaccines or vehicles to deliver therapeutic drugs to the target site in the body,« explains Uetrecht. Usually, norovirus shells contain 180 units of the VP1 protein. However, some virus-like particles consist, for example, of 60 units. In mass-spectrometry studies, the researchers have found out what conditions are necessary for the formation of different particle sizes. It‘s crucial to be able to create uniform sizes so that the particles are suitable as reliable vaccines or transporters of drugs.
Although the analysis of viruses using native mass spectrometry is successful and extremely complex, Uetrecht calls this part of her activities »bread-and-butter work«. What she means is that it gives her findings she can publish and that prove her status as a competent researcher. The other part of her work consists of stubbornly pursuing an idea she had in 2010, but that takes a lot of time and work to implement.
Filtering molecules for X-rays
Back then, she was writing her doctoral thesis in the Netherlands. She read an article by Swedish colleagues because they had cited one of her publications. The introduction to the article mentioned a method of taking snapshots of proteins and protein complexes with short lifetimes using ultrashort pulses of laser light in the X-ray range. Compared to native mass spectrometry, these snapshots reveal much more information about the structure of the giant molecules. However, Uetrecht realized immediately that the evaluation of this single-particle imaging would be more difficult the more different proteins, and giant molecules were present in the sample. She also knew that the existence of long-lived molecules alongside the short-lived molecules would cause problems. »Then I had the idea of using native mass spectrometry to filter out co-existing, short-lived protein states according to mass and then channeling them directly into the X-ray laser beam,« says Uetrecht.
In 2010, X-ray laser technology was still largely theory. It was only in 2017 that the first European system of this kind was commissioned in Schenefeld near Hamburg. This was the European XFEL (X-Ray Free-Electron Laser). To generate the X-ray pulses, electrons, initially in packages, are accelerated in a 1.7-kilometer underground accelerator to high energies and speeds. Then, special magnetic structures channel the speeding electrons into a narrow, wave-like course. Each individual electron emits X-ray light which is increasingly amplified. The 3.4-kilometer facility cost EUR 1.2 billion (at 2005 prices).
During the construction of the European XFEL, Uetrecht already developed her idea of the combination of native mass spectrometry and individual particle imaging into a single concept. At the same time, she searched for funding. Today, she is the coordinator of the EU project MS SPIDOC, which aims to prove the practicability of the concept. She and her colleagues have their offices and laboratories in the Centre for Structural Systems Biology (CSSB) very near the European XFEL, although they work for the University of Siegen. Uetrecht hopes that, at the beginning of 2023, the combined mass spectrometry X-ray laser system at the European XFEL is supposed to deliver the first images of assembled shell/capsid proteins. Based on these images, it should be possible to later reconstruct the structure of each protein atom-by-atom in just a short time. Then, Uetrecht and her team will be within touching distance of their goal of precisely analyzing the norovirus in order to effectively combat it.
These products might interest you
See the theme worlds for related content
Topic World Mass Spectrometry
Mass spectrometry enables us to detect and identify molecules and reveal their structure. Whether in chemistry, biochemistry or forensics - mass spectrometry opens up unexpected insights into the composition of our world. Immerse yourself in the fascinating world of mass spectrometry!

Topic World Mass Spectrometry
Mass spectrometry enables us to detect and identify molecules and reveal their structure. Whether in chemistry, biochemistry or forensics - mass spectrometry opens up unexpected insights into the composition of our world. Immerse yourself in the fascinating world of mass spectrometry!