How a CRISPR protein might yield new tests for many viruses
In a first for the genetic toolset known as CRISPR, a recently discovered protein has been found to act as a kind of multipurpose self-destruct system for bacteria, capable of degrading single-stranded RNA, single-stranded DNA and double-stranded DNA. With its abilities to target so many types of genetic material, the discovery holds potential for the development of new inexpensive and highly sensitive at-home diagnostic tests for a wide range of infectious diseases, including Covid-19, influenza, Ebola and zika, according to the authors of a new study in the journal Nature.
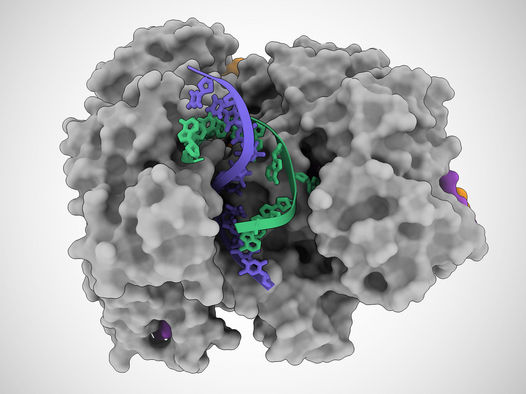
In this illustration based on cryo-electron microscope images, a Cas12a2 protein unzips a DNA double helix, allowing it to cut the single strands of DNA (blue and green).
Jack Bravo/University of Texas at Austin
Using a high-resolution imaging technique called cryo-EM, the team discovered that when this protein, named Cas12a2, binds to a specific sequence of genetic material from a potentially dangerous virus, called a target RNA, a side portion of Cas12a2 swings out to reveal an active site, similar to a sprung-open switchblade knife. Then, the active site starts to indiscriminately cut any genetic material it comes into contact with. The researchers discovered that, with a single mutation to the Cas12a2 protein, the active site degrades only single-stranded DNA—a feature especially useful in developing new diagnostics tailored for any of a wide range of viruses.
A test based on this technology could theoretically combine the best features of PCR-based tests that detect genetic material from a virus (high sensitivity, high accuracy and the ability to detect an active infection) with the best features of rapid at-home diagnostic tests (inexpensive to produce without requiring specialized lab equipment). It also would be easily adaptable to any new RNA virus.
“If some new virus comes out tomorrow, all you have to do is figure out its genome and then change the guide RNA in your test, and you’d have a test against it,” said David Taylor, an associate professor of molecular biosciences at The University of Texas at Austin and co-corresponding author of the new study.
Such a diagnostic would still require separate work and probably involve collecting saliva or a nasal sample from a patient to be mixed with the team’s modified Cas12a2 protein, the piece of guide RNA that acts like a mugshot to identify a specific virus, and a fluorescent probe designed to light up when its single-stranded DNA gets cut.
CRISPR is the name for a set of tools that occur naturally in bacteria, but which scientists have adapted for use in gene editing. This is the first CRISPR protein that has been found to degrade such a wide range of genetic material.
“Cas12a2 basically grabs the two ends of the DNA double helix and bends it really tightly,” said Jack Bravo, a postdoctoral fellow at UT Austin and co-first author on the paper. “And so, the helix in the middle pops open, and then this allows this active site to destroy the bits of DNA that become single-stranded. This is what makes Cas12a2 different from all the other DNA-targeting systems.”
Original publication

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.