Antibiotic ribosomal compounds deciphered
Combating multidrug-resistant bacteria through high-resolution structural imaging
A European research team led by the Department of Chemistry at the University of Hamburg presents structures of 17 different antibiotic ribosomal compounds with high detail resolution in a study. The knowledge could pave the way for the development of novel antibiotics to combat multidrug-resistant bacteria.
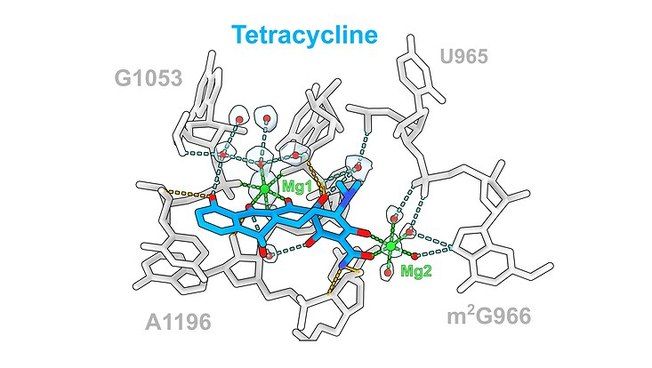
Antibiotic tetracycline (blue) and its interactions with ribosomal rRNA Photo: UHH/Paternoga The antibiotic tetracycline (blue) and its interactions with ribosomal rRNA (gray), which are mediated via direct (orange) or indirect (cyan) interactions via Mg1 (green) or water molecules (red).
UHH/Paternoga
Ribosomes are large molecular complexes found in the cells of plants, animals, humans as well as bacteria that produce proteins. Therefore, they are important targets for antibiotics, which in bacteria couple to a subunit of the ribosomes and cause reading errors or even reading stops. This results in the formation of defective proteins that lose their biological function, and the bacterium dies. However, more and more multi-resistant bacteria can prevent antibiotics from working.
To circumvent these resistances, a detailed understanding of antibiotics is essential, especially antibiotic ribosome structures. Over the past two decades, antibiotic ribosome structures for each major class of ribosome-directed antibiotics have been published, providing insights into their binding sites and mechanisms of action.
The team led by Prof. Daniel N. Wilson, Ph.D., of the Department of Chemistry at the University of Hamburg, Germany, has now used cryo-electron microscopy to image these structures at a resolution of 1.6 to 2.2 Ångstroms (Å), where one Å corresponds to the ten-millionth part of a millimeter. "In the past, antibiotic ribosome structures were mainly presented at resolutions of 2.5 to 3.5 Å," said Prof. Daniel N. Wilson, PhD. "The improved resolution now allows us to observe even water molecules that support drug-drug interactions." The researchers found that generally ten to 20 hydrogen bonds interacted with the ribosome, with the contribution of water molecules varying for different classes of antibiotics.
The antibiotics studied included the six clinically relevant antibiotic families, including the tetracyclines used for respiratory infections, aminoglycosides with a broad spectrum of activity, including tuberculosis or meningitis, and the pleuromutilins for skin and soft tissue infections.
For the study, the research team applied these antibiotics to so-called 70S ribosomes of the bacterium "Escherichia coli" - a coliform bacterium that is also found in the human intestine. "We anticipate that this information can be used in the future for structure-based design of new antibiotic derivatives. By identifying regions that can be altered, the bound water can be selectively displaced to take on an interaction with the target, which is the bacterium," Wilson says.
The high-resolution imaging is a big step, according to Wilson: "The old antibiotic ribosome structures at lower resolution show that the binding sites for each class of antibiotic are similar, but in many cases there are profound differences in terms of the exact location of the drugs as well as the drug-binding site." Therefore, he said, the high-resolution experimental data were needed to provide a more accurate description of the interactions of antibiotics with the ribosome.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.




























































