USC scientists generate first detailed map of human neuroreceptor
Research could pave the way for development of better drugs to target neurodegenerative diseases
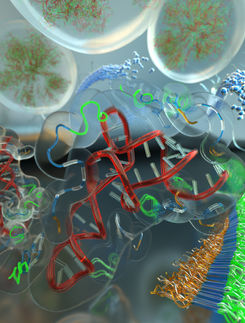
For the first time, USC scientists have mapped out a neuroreceptor. This scientific breakthrough promises to revolutionize the engineering of drugs used to treat ailments such as Alzheimer's disease and schizophrenia.
The team produced the world's first high-resolution images of the α7 (Alpha 7) receptor, a molecule responsible for transmitting signals between neurons – particularly in regions of the brain believed to be associated with learning and memory.
Using the image, scientists will be better equipped to design pharmaceuticals specifically to interact with the receptor, instead of blindly using a trial-and-error approach.
"A lot of interest in this work will come from pharmaceutical companies," said corresponding author Lin Chen, professor of biological sciences and chemistry at the USC Dornsife College of Letters, Arts and Sciences. "They really have no clear picture of this. They don't know how or why [their drugs] work."
The high-resolution image will also help neuroscience researchers study how these receptors receive and transmit neuronal signals, a question that has puzzled researchers for decades.
The article, co-authored with scientists from the Keck School of Medicine of USC and the Mayo Clinic College of Medicine, appears in Nature Neuroscience.
Developing an image of the α7 receptor was no simple task, which is partly why it has taken until now to achieve this despite the wide interest in the understanding the receptor's structure. Attempts to decipher neuroreceptors have been ongoing for 30 years.
"This has been a longstanding challenge," Chen said. The challenge is twofold, he said. It is difficult to obtain enough receptor protein for structural analysis, and the flexible nature of these receptors makes them difficult to crystallize — a necessary step for high-resolution imaging.
The biologist's usual go-to method to study such molecules — growing a large quantity using molecular cloning — failed to produce enough correctly structured α7 to study.
"You can't study it directly in its natural form, so you have to engineer it," Chen said.
In the case of α7, Chen's collaborator, Dr. Steve Sine from Mayo Clinic, engineered a chimera, a Frankenstein molecule sharing about 70 percent of its structure in common with the α7 that reacted to stimuli in the same way that natural α7 does.
The next step was to form crystals with these proteins for high-resolution study. This turns out to be particularly difficult for neuronal receptors because they are intrinsically flexible — they need to bind to a neurotransmitter, a small molecule that acts as a messenger in the nervous system, and transmit the signal across the protein body. Moreover, these receptors are decorated with sugar molecules that add further flexibility to the system.
The crystallization of α7 was a painstaking process carried out by Shu-xing Li, the first author of the study and a postdoctoral fellow in Chen's lab. For every hundred crystals obtained, only one or two were good enough for structural analysis. Li had to sort through hundreds of crystals to collect enough data for structural analysis.
"In a sense, these crystals are probably among the world's most expensive crystals, certainly more expensive than diamond," Chen said. "But considering the rich information we can get from these crystals about human neuronal receptors, and the potential impact on drug development that can benefit human health, they are worth the effort."
Organizations
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Nanotube-based sensors can be implanted under the skin for a year
Drug Development Technology: Frost & Sullivan Award goes to Imperacer
Scientists Develop the First Atomic View of Key Genetic Processes
A Powerful New Tool for Decoding Gene Functions in Mammals and Man
Giant panda genome reveals new insights into the bear's bamboo diet