Lab-made complexes are “sun sponges”
A ring of protein and pigments, half synthetic and half natural, can be used to quickly prototype light-harvesting antennas that absorb more sunlight than fully natural ones
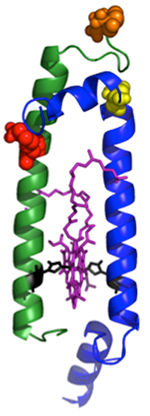
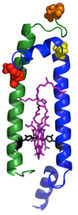
Antennas are made up of modules: a two-peptide dyad (side view shown above) with the pair of bacteriochlorophyll molecules (purple) in the middle. The bacteriochlorophylls absorb light and trap the energy transferred from other pigments. The additional pigmentsare attached at carefully chosen sites (red, orange and yellow) on the beta peptide (the green helix), or the top end of the alpha peptide (blue helix).
COGDELL/Holten/PARC
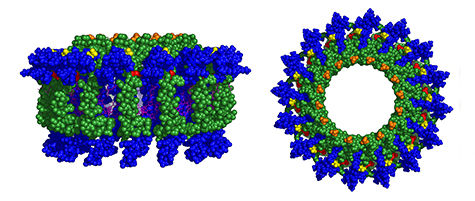
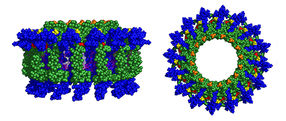
The dyad modules assemble into rings (side view, above left, and top view, above right) that pack many pigments together for light harvesting. Because of the additional pigments attached to the dyads, the antennas absorb more of the sun’s light than natural systems.
HUNTER/Holten/PARC
In diagrams it looks like a confection of self-curling ribbon with bits of bling hung off the ribbon here and there. In fact it is a carefully designed ring of proteins with attached pigments that self-assembles into a structure that soaks up sunlight.
The scientists who made it call it a testbed, or platform for rapid prototyping of light-harvesting antennas–structures found in plants and photosynthesizing bacteria–that take the first step in converting sunlight into usable energy. The antennas consist of protein scaffolding that holds pigment molecules in ideal positions to capture and transfer the sun’s energy. The number and variety of the pigment molecules determines how much of the sun’s energy the antennas can grab and dump into an energy trap.
In the online edition of Chemical Science the scientists describe two prototype antennas they’ve built on their testbed. One incorporated synthetic dyes called Oregon Green and Rhodamine Red and the other combined Oregon Green and a synthetic version of the bacterial pigment bacteriochlorophyll that absorbs light in the near-infrared region of the spectrum.
Both designs soak up more of the sun’s spectrum than native antennas in purple bacteria that provided the inspiration and some components for the testbed. The prototypes were also far easier to assemble than synthetic antennas made entirely from scratch. In this sense they offer the best of both worlds, combining human synthetic ingenuity with the repertoire of robust chemical machinery selected by evolution.
One day a two-part system (consisting of an antenna and a second unit called a reaction center) might serve as a miniature power outlet into which photochemical modules could be plugged. The sun’s energy could then be used directly to split water, generate electricity, or build molecular-scale devices.
Designer pigments
Nature has evolved many different systems to capture the sun’s energy, but they all rely on pigments, molecules that appear strongly colored because they are selectively absorbing some wavelengths, or colors, of light in the solar spectrum.
The pigment we are most familiar with is chlorophyll, the molecule that makes plants appear green. But that green color is a tipoff about the plant’s solar absorption. We see plants as green because they’re reflecting the green part of the spectrum and absorbing in the violet and the red parts of the spectrum instead.
Not only do plants miss the middle of the visible spectrum, they also miss light at wavelengths longer than we can see, including near-infrared photons absorbed by photosynthetic bacteria. The accessory pigments such as carotenoids that give leaves their splendid fall colors fill some gaps but large swaths of the solar spectrum pass through untouched.
“Since plant pigments actually reject a lot of the light that falls on them,” Hunter said, “potentially there’s a lot of light you could gather that plants don’t bother with.”
The team relies on Jonathan Lindsey to design and synthesize pigments that can absorb at wavelengths that will fill some of the holes in the absorption of natural systems. “It can’t be done from first principles,” Lindsey said, “but we have a large database of known absorbers and so drawing on that and reasoning by analogy we can design a large variety of pigments.”
More than one synthetic or natural pigment can be attached to the protein scaffolding. “The prototypes in the Chemical Science paper both have two but ultimately we’d like to add three or four or even more,” said Lindsey. “One of our goals is to understand to what extent the protein can be derivatized with pigments.”
“The effectiveness of the design depends not only on having extra pigments but also pigments able to talk to one another, so that energy that lands on any one of them is able to hop onto the next pigment and then to the next one after that. They have to work together,” Hunter explained.
“The energy cascades down like a waterfall,” Hunter said. “So you pour the energy at the top of the waterfall and it hits one pigment and jumps to the next and the next and finally to the pigment at the bottom, which in terms of energy is the pigment that is reddest in color.”
Self assembly line
If broad spectral coverage was one goal of the project, another was to avoid the laborious synthesis typically required to make designer light-harvesting antennas.
Fortunately light-harvesting antennas from purple bacteria are modular devices that self assemble under appropriate conditions, conditions that have been worked out by team members Paul Loach and Pamela Parkes-Loach. The basic module is a pair of peptides (short proteins) called alpha and beta that in turn house two bacteriochlorophyll molecules that both absorb light and act as the trap for all the harvested energy.
Thanks to the chemical affinities of the components, they self-assemble into dyads when added together in detergent (detergent is used instead of water alone because parts of the peptides shun water). By adjusting the detergent concentration and temperature, the dyads form rings, which in native antenna contain up to 16 alpha/beta dyads and thus as many as 32 bacteriochlorophylls.
In the testbed, the scientists use peptides that have been slightly modified from the native amino acid sequence for attachment of the extra pigments to increase solar spectral coverage. The attachment sites were chosen to avoid disrupting the self assembly of the components into dyads and dyads into rings.
“This is an example of what the field would refer to as semi-synthesis,” Lindsey said. “We take naturally occurring materials and combine them with synthetic ones to make something that doesn’t exist in nature. By taking lots of material from nature we can make molecules that are architecturally more complex than those we can make from scratch.”
Once assembled, the antenna are sent to the Holten/Kirmaier lab, where a variety of spectroscopic methods including ultra-fast laser spectroscopy are used to excite each pigment molecule and to follow the energy transfer from one pigment to the next and down to the target bacteriochlorophyll. Given the right pigments in the right locations, this transfer is extremely efficient, and little energy is lost on the way.
Samples also went to the Bocian laboratory where they are probed for structural integrity and to the Hunter laboratory where images are made of the rings, which are only 11 to 16 nanometers (a billionth of a meter) across and must be magnified tens of thousands of times to be visible.
“I’ve been working in photosynthesis for 50 years,” said Loach, “and I can’t think of many other times when there were so many good people with so many different talents coming together to try to solve problems. It’s fun to be part of it and to see what comes out of the collaboration.”