Shape-sifting: NIST categorizes bio scaffolds by characteristic cell shapes
Getting in the right shape might be just as important in a biology lab as a gym. Shape is thought to play an important role in the effectiveness of cells grown to repair or replace damaged tissue in the body. To help design new structures that enable cells to "shape up," researchers at the National Institute of Standards and Technology (NIST) have come up with a way to measure, and more importantly, classify, the shapes cells tend to take in different environments.
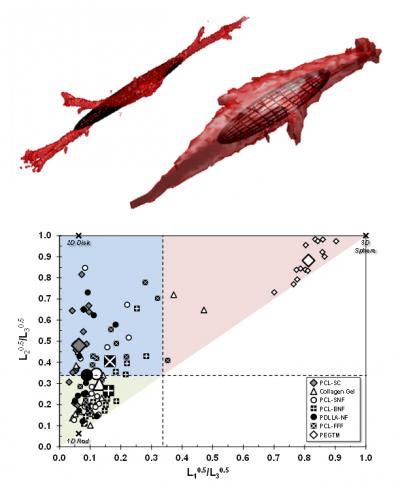
(Top) The same basic cell type grown on two different bio scaffolds (collagen gel and a grid-like scaffold made of a biocompatible polymer) adopts significantly different shapes. The superimposed ellipsoids are calculated from the dimensions of each cell. (Bottom) Plotting the characteristic ellipsoids for each cell by how round they are in the two major cross sections reveals that cells tend to different shapes on different scaffolds -- spheres at one extreme, long narrow rods at another.
Farooque,Camp,Simon/NIST
With the notable exception of Flat Stanley, we all live, and are shaped by, a 3-dimensional world. Biologists have accepted that this dimensional outlook is just as important to growing cells. A key challenge in tissue engineering—the engineering of living cells to grow into replacement or repair tissues such as bone, heart muscle, blood vessels or cartilage—is creating 3-D scaffolds to support the cells as they grow and provide an appropriate environment so that they develop into viable tissue.
This, says NIST materials scientist Carl Simon, has led to a large and rapidly expanding collection of possible 3D scaffolds, ranging from relatively simple gels made of collagen, the body's natural structural matrix, to structured or unstructured arrangements of polymer fibers, hydrogels and many more.
"What we're trying to measure," Simon explains, "is 'what is 3D in this context?' Presumably, a scaffold provides some sort of microenvironment—a niche that allows a cell to adopt the normal 3D morphology that it would have in the body. But you can't measure the niche because that's sort of an amorphous, ill-defined concept. So, we decided to measure cell shape and see how that changes, if it becomes more 3D in the scaffold."
The NIST team made painstaking measurements of individual cells in a variety of typical scaffolds using a confocal microscope, an instrument that can make highly detailed, 3-dimensional images of a target, albeit with very lengthy exposure times. They then used a mathematical technique—"gyration tensors"—to reduce each cell's shape to a characteristic ellipsoid. Ellipsoids can range in shape from points or spheres to flat ellipses or elongated sticks to something like an American football.
Analyzing the ellipsoid collection allowed them to categorize average cell shapes by scaffold. Cells in collagen gels and some fiber scaffolds, for example, tend toward a 1-dimensional rod shape. Other scaffolds promoted 2-dimensional disks, while a synthetic gel using a material called PEGTM seems to encourage spheres.
"This technique," says Simon, "gives you a way to compare these different scaffolds. There are hundreds of scaffolds being advanced. It's hard to know how they differ with respect to cell morphology. By looking at the cell shape in 3D with this approach, you can compare them. You can say this one makes the cells more 3-dimensional, or this one makes the cells more like they would develop in collagen, depending on what you want. "
Original publication

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.