Fast-moving cells walk in a stepwise manner
A team of biologists and engineers at the University of California, San Diego has discovered that white blood cells, which repair damaged tissue as part of the body's immune response, move to inflamed sites by walking in a stepwise manner. The cells periodically form and break adhesions mainly under two "feet," and generate the traction forces that propel them forward by the coordinated action of contractile proteins. Their discovery in the Journal of Cell Biology, is an important advance toward developing new pharmacological strategies to treat chronic inflammatory diseases such as arthritis, irritable bowel syndrome, Type 1 diabetes, and multiple sclerosis.
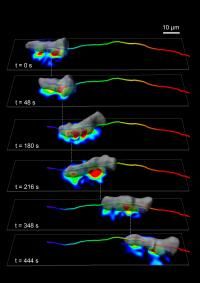
A team of biologists and engineers at UC San Diego applied advanced mathematical tools to answer a basic question in cell biology about how cells move and discovered that the mechanism looks very similar to walking. Starting with an amoeboid Dictyostelium cell, the team found the cell periodically forms and breaks adhesions mainly under two "feet," and generates the traction forces (indicated by the heatmap) that propel them forward by the coordinated action of contractile proteins. The researchers then applied the same technology to study human white blood cells, which are responsible for moving to sites of infection and inflammation to repair tissue, and found the same "walking" mechanism at work. Their discovery, published March 17 in the Journal of Cell Biology, is an important advance toward developing new pharmacological strategies to treat chronic inflammatory diseases, which are caused by the uncontrolled recruitment of white blood cells to the site of inflammation.
Image courtesy of the Journal of Cell Biology and ©2014 Bastounis et al.
"The immune system requires the migration of white blood cells to the point of infection and inflammation to clear invaders and begin the process of digesting and repairing tissue. However, when the body fails to properly regulate the recruitment of these cells, the inflammation can become chronic resulting in irreversible tissue injury and loss of functionality," said Juan C. Lasheras, a professor in the departments of Mechanical and Aerospace Engineering and Bioengineering, and in the Institute for Engineering in Medicine. "Understanding the way in which these cells generate the necessary forces to move from the blood stream to the site of inflammation will guide the design of new strategies that could target specific mechanical processes to control their migration," Lasheras said.
Figuring out how white blood cells move required an interdisciplinary approach involving engineering and biological sciences. The lead author of the study is Effie Bastounis, a member of a team led by UC San Diego Jacobs School of Engineering professors Lasheras and Juan Carlos del Alamo, of the Department of Mechanical and Aerospace Engineering, and Richard A. Firtel, a professor of Cell and Developmental Biology in the Division of Biological Sciences. "This work was made possible through interdisciplinary approaches that applied mathematical tools to a basic question in cell biology about how cells move," stated Richard Firtel. "By first applying novel methodologies to study the amoeba Dictyostelium, an experimental system often used by cell biologists, we were able to discover the basic mechanisms that control amoeboid movement, which we then applied to understanding white blood cells."
The team used new analytical tools to measure, with a high degree of accuracy and resolution, the forces the cells exert to move forward. The novel methodology, which they have been refining during the last several years supported by grants from the National Institutes of Health (R01-GM084227 and R01-GM037830), is called Fourier Traction Force Microscopy. Before their study, scientists thought white blood cells did not move in a highly coordinated manner. Furthermore, their work discovered that cells move by not only extending themselves at their front and contracting their backs, but also by squeezing inwardly along their lateral sides pushing the front of the cell forward. These findings establish a new paradigm as to how cell move. The research team is currently extending their techniques, which they have used to study leukocytes and other types of amoeboid cells, to investigate the mechanics of cancer cell migration and invasion.
Most read news
Topics
Organizations

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.