How intestinal cells build nutrient-absorbing surface
The "brush border" – a densely packed array of finger-like projections called microvilli – covers the surfaces of the cells that line our intestines.
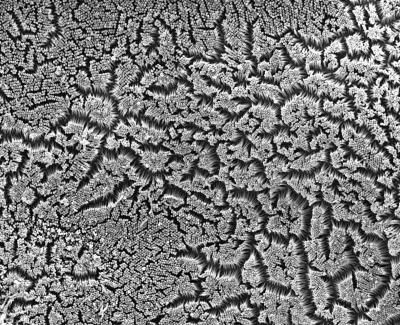
Matthew Tyska, Ph.D., and colleagues, Vanderbilt University
Vanderbilt University researchers have now discovered how intestinal cells build this specialized structure, which is critical for absorbing nutrients and defending against pathogens. The findings, published in the journal Cell, reveal a role for adhesion molecules in brush border assembly and increase our understanding of intestinal pathologies associated with inherited and infectious diseases.
Pathogens that destroy the intestinal brush border cause diarrhea and death – a particular problem in communities that do not have safe water supplies.
"Without a fully functional brush border that has enough surface area and absorptive capacity, you can't survive," said Matthew Tyska, Ph.D., associate professor of Cell and Developmental Biology and senior author of the Cell paper. "Our basic science discoveries provide a framework for thinking about how to control intestinal surface morphology – and how to repair it."
Despite the essential role of the brush border, its assembly has remained a mystery. Using scanning electron microscopy, Tyska and postdoctoral fellow Scott Crawley, Ph.D., followed the generation of the brush border over time in an epithelial cell culture model.
They found that as the microvilli begin to emerge, they stick to each other at their tips and form small teepee-like clusters. Over time, these clusters grow to include more and more microvilli, until the entire cell surface is covered by microvilli of uniform height.
"This was really the fundamental observation that gave rise to the rest of the project – that these microvilli come out of the cell surface and stick together at the tips," Tyska said.
When the investigators closely examined the electron micrographs, they saw thread-like links connecting microvilli at their tips. One of their collaborators imaged intestinal tissue and found the same links – a "web of connections" between the microvilli, Tyska said.
"We were excited by the idea that physical adhesion between microvilli might provide the driving force for growing and tightly packing the brush border," Tyska said.
The investigators suspected that proteins in the cadherin family – calcium-dependent adhesion molecules that allow cells to stick together – might mediate the interaction between microvilli. They had previously used proteomics technologies to define all of the proteins in the isolated brush border. The list included two candidates for microvillar tip adhesion: protocadherin-24 and mucin-like protocadherin.
In a series of studies, the team demonstrated that these two cadherins have a role in sticking microvilli together. Experimental reduction of protocadherin-24 in the cell culture model destroyed the brush border. Microvilli still formed, but they were not tightly packed and they had variable lengths.
"It's always been a question how microvilli achieve this remarkably uniform length," Tyska said. "Now it looks like the solution is simple – they get tied together at the tips and one can't get past another. It's really straightforward."
In biochemical experiments, the researchers discovered that the cadherin molecules interacted with two proteins inside microvilli – a motor protein connected to the cell cytoskeleton and an adapter protein called harmonin. These connections were required for the localization of the cadherins to the microvillar tips.
Interestingly, harmonin had been studied by other investigators for its role in linking protrusions on the inner ear hair cell – a sensory structure involved in hearing. Genetic mutations that disable harmonin cause Usher syndrome, a form of inherited deaf-blindness. Patients with Usher syndrome also have poorly characterized intestinal disease.
Tyska and his colleagues realized that harmonin mutations might disrupt the intestinal microvillar tip adhesion complex and destabilize the brush border. In a mouse missing harmonin, a model of Usher disease, they found poorly formed brush border in some parts of the intestines and no brush border in other areas.
"We think that patients with Usher disease probably have issues with brush border ultrastructure that are causing problems with homeostasis and leading to intestinal symptoms," Tyska said.
"Making the initial electron microscopy discovery, defining the molecular mechanism and then connecting it to human disease – that's a scientific hat trick," he said.
Tyska and his colleagues are exploring whether a similar adhesive complex guides the assembly of brush border structures in other epithelial tissues, such as the kidneys.