High-speed snapshots of biomolecules
PETRA III pioneers protein serial crystallography at synchrotrons
Using DESY's synchrotron light source PETRA III, scientists have pioneered a new way to analyse delicate biomolecules. The novel approach, borrowed from a new class of high-intensity X-ray sources called free-electron lasers (FELs), could reveal the atomic structure of proteins that were previously inaccessible to synchrotrons, as the team led by Prof. Henry Chapman from the Hamburg Center for Free-Electron Laser Science CFEL reports in the scientific journal of the International Union of Crystallography, IUCrJ. CFEL is a joint institution of DESY, the University of Hamburg and the Max Planck Society.
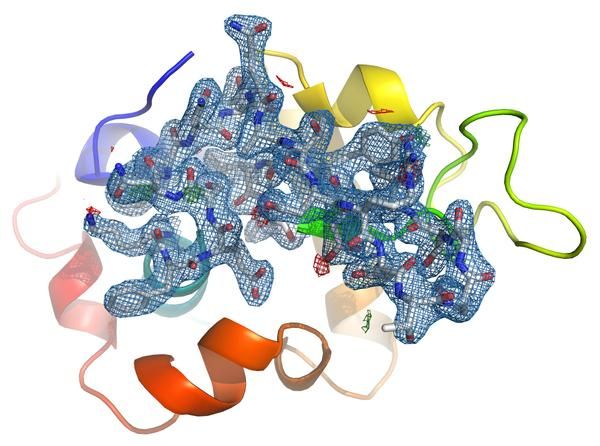
Electron density map of lysozyme at 2.1 Å resolution calculated from 40,233 single crystal indexed diffraction patterns.
DESY
The atomic structure of biomolecules can reveal the mechanisms underlying their function in the organism, leading to improved biological insight with the potential to enable the development of new medicines. The standard technique for elucidating the atomic structure of proteins involves shining a bright beam of X-rays onto a protein crystal. The crystal scatters the X-rays in a characteristic way, and from the resulting diffraction pattern the inner structure of the crystal can be calculated, yielding the atomic structure of the protein.
"But being jammed into a crystal is not a natural state for most biomolecules, so, many proteins form only very tiny crystals," says DESY scientist Chapman,who is also a professor at the University of Hamburg and a member of the Hamburg Center for Ultrafast Imaging CUI. “The smaller the crystal, the brighter the X-ray light has to be in order to produce usable diffraction patterns.”
Free-electron X-ray lasers can analyse crystals even smaller than one micrometre across, but a single X-ray pulse vaporises the sample after giving a single snapshot. Measurements are made from many tens of thousands of samples in quick succession, and then processed with high performance computers. Synchrotrons on the other hand can determine the atomic structure from just a single crystal, but it has to be large enough and of high quality to be able to rotate it through the beam and explore it from all angles. Often, a handful of high-quality crystals is used.
"We noticed at the X-ray laser that some of the samples were large enough and diffracting quite well, so that we sometimes even had to attenuate the beam so as not to burn the detector," explains first author Dr. Francesco Stellato from CFEL. "So moving the quick-succession FEL method to a bright synchrotron could maybe serve to close a size gap for samples that are too small for conventional synchrotron investigations and sort of too large for free-electron lasers."
To test their hypothesis, the scientists used a well-studied protein called lysozyme and grew micrometre-sized crystals of it. At PETRA III, currently the most brilliant synchrotron light source in the world, they took FEL-style high-speed snapshots of many tiny lysozyme crystals and combined them. “We let a small stream of microcrystals run across the X-ray beam and shot a series of pictures as fast as the detector would allow,” illustrates CFEL scientist Dr. Dominik Oberthür from the team.
The lysozyme crystals were fed as a suspension in water through a thin capillary across the nine micrometre wide X-ray beam. This technique works at room temperature, avoiding the need to deep-freeze the protein crystals. The flow speed was adjusted so that individual microcrystals would not suffer significant radiation damage during the passage. The transit time of the microcrystals across the beam was between one and three milliseconds. “We set the detector on rapid fire, taking 25 pictures per second,” says co-author Dr. Alke Meents, head of the measuring station P11, where the experiments took place.
Almost 1.5 million detector frames were acquired over about 17 hours of experimental time. Automated analysis routines sorted through all the frames to find the 40,233 diffraction patterns hidden amongst them, which were then combined for structural analysis. This yielded the lysozyme structure with a resolution of 2.1 Ångström, or 0.2 millionths of a millimetre and in excellent agreement with known structural data. “With planned improvements on brightness and detectors, even faster images can be recorded, increasing the spatial resolution,” underlines Meents. “This way, our work paves the way for structural analysis of biomolecules previously inaccessible by synchrotrons.”
The work, that was funded by the German Federal Ministry of Education and Research BMBF in the context of the Röntgen-Ångström-Cluster, followsanother recent explorationof porting FEL techniques to the synchrotron on cryogenically cooled samples that were mounted in a nylon loop and scanned with the fine X-ray beam of PETRA III.
Original publication
See the theme worlds for related content
Topic world Protein analytics
Protein analytics provides a deep insight into these complex macromolecules, their structure, function and interactions. It is essential for discovering and developing biopharmaceuticals, understanding disease mechanisms, and identifying therapeutic targets. Techniques such as mass spectrometry, Western blot and immunoassays allow researchers to characterize proteins at the molecular level, determine their concentration and identify possible modifications.

Topic world Protein analytics
Protein analytics provides a deep insight into these complex macromolecules, their structure, function and interactions. It is essential for discovering and developing biopharmaceuticals, understanding disease mechanisms, and identifying therapeutic targets. Techniques such as mass spectrometry, Western blot and immunoassays allow researchers to characterize proteins at the molecular level, determine their concentration and identify possible modifications.