'Gene fusion' mutation uses 3-way mechanism to drive childhood brain cancers
A powerful, three-way mechanism by which a mutation drives the growth of childhood brain cancers, was discovered by scientists from the Perelman School of Medicine at the University of Pennsylvania and The Children's Hospital of Philadelphia (CHOP). The team hopes the discovery will lead to better methods for diagnosing and treating these cancers, which cannot always be cured with surgery.
Three-Way mechanism drives pediatric low-grade gliomas.
Payal Jain and Adam Resnick, Perelman School of Medicine, University of Pennsylvania
"Treating pediatric brain cancer patients with the wrong chemotherapy can cause lasting harm, and this finding should enable us to avoid that in many cases through a more accurate diagnosis," said study co-leader Adam C. Resnick, PhD, an assistant professor of Neurosurgery at Penn Medicine. He expects that the finding also will lead to discoveries of similar tumor-driving mechanisms in other forms of cancers in both children and adults.
The study emerged from a multi-center collaborative effort led by Penn, CHOP, and the Dana Farber Cancer Institute. The broad aim of the collaboration has been to gain a more detailed understanding of the genomic abnormalities underlying the most common childhood brain cancers, known as pediatric low-grade gliomas.
Researchers already know that certain sub-types of these gliomas are driven mostly by mutations or duplications to genes in the growth-related, mitogen-associated protein kinase (MAPK) pathway. Drugs that block MAPK signaling are beginning to be investigated in clinical trials with pediatric patients. In this study, however, Resnick and his colleagues sought to characterize abnormalities in pediatric gliomas sub-types that are not driven by MAPK-pathway mutations.
With dozens of laboratories collaborating in the study, the team was able to put together the largest-ever genomic dataset from these rare tumors, comprising 249 cases. They found that besides MAPK-related alterations the most common genetic abnormalities were those involving a growth-driving gene, MYB, which normally is active only during the fetal phase of life.
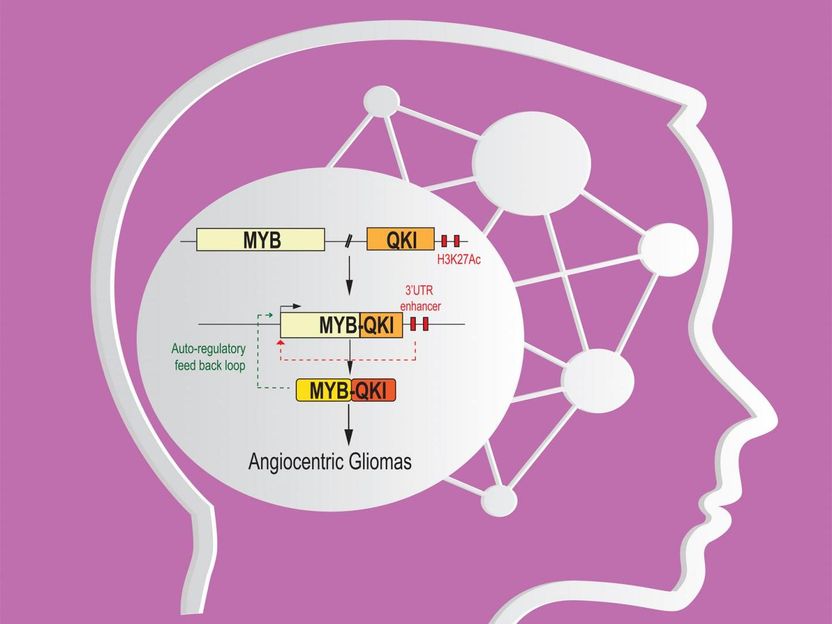
The MYB alterations were especially frequent in one particularly rare pediatric glioma subtype, angiocentric glioma. In fact, all of the 19 angiocentric glioma samples had detectable MYB alterations, and there was evidence that most of these alterations involved one specific abnormality: a deletion of chromosomal DNA that left one end of MYB fused to a normally separate gene, QKI.
Investigating further, the team found that the MYB-QKI gene fusions appeared to be driving these brain tumors in three different ways.
First, although MYB is normally not expressed in the developed human brain, the gene arrangement in angiocentric gliomas leads to abnormal expression and transcriptional activity of MYB via the MYB-QKI gene fusion--and indeed the fused MYB-QKI works as an oncogene to drive tumor formation.
Second, the fusion deletes enough of QKI to disrupt its chief function, which appears to be tumor suppression, thus QKI fails to exert its usual braking effect when the rate of cell division becomes aberrant.
The third, most new and interesting mechanism turned out to involve a combination of elements from the two genes. "We found that the fusion event brings QKI-related enhancer elements close enough to MYB's promoter region to activate it, thereby driving MYB-QKI expression," said Payal Jain, a PhD student in the Cell and Molecular Biology program at Penn and working in Resnick's laboratory. In addition, MYB-QKI can drive its own expression, propelling a positive feedback loop in these tumors.
Taking all of this together, a cell attempting to protect itself from excessive growth by dialing up QKI's usual growth-slowing activity would instead boost the expression of the fused MYB-QKI with its overall growth-promoting activity. Experiments in mice confirmed that the MYB-QKI fusion in injected test cells is sufficient to create tumors.
In the short term, the findings should enable physicians to identify pediatric gliomas that contain these mutations, thus sparing some children MAPK-targeted therapy that may not work. The discovery also suggests strategies for future new therapies--for example, blocking the epigenetic dysregulation that helps drive MYB-QKI activity.
Such therapies may end up being applicable to a much wider range of cancers. "Gene fusions are common in cancers, and we think it's likely that some will be found to work via combinatorial mechanisms like those we've uncovered in this study," Resnick said.
Jain, Resnick, and their colleagues will follow up with investigations of other gene fusions and abnormalities found in pediatric gliomas in the hope that specific treatments can be developed for all subtypes of these cancers.
"Pediatric brain tumors are rare, so it's only through large collaborations like this one that we can bring together enough samples and data to do such meaningful studies," Resnick said. Collaborating with Resnick on the project was Phillip Storm, MD, an associate professor of Neurosurgery and Neurosurgery division chief at CHOP. Together they co-direct the Center for Data Driven Discovery in Biomedicine at CHOP.