Controlling ultrafast electrons in motion
An international team has used the light produced by the Free Electron Laser FERMI at the research Centre Elettra Sincrotrone Trieste in the AREA Science Park to control the ultrafast movement of electrons. The experiment opens the way to the study of more complex processes which occur in nature on the scale of attoseconds (billionths of a billionth of a second), such as photosynthesis, combustion, catalysis and atmospheric chemistry.
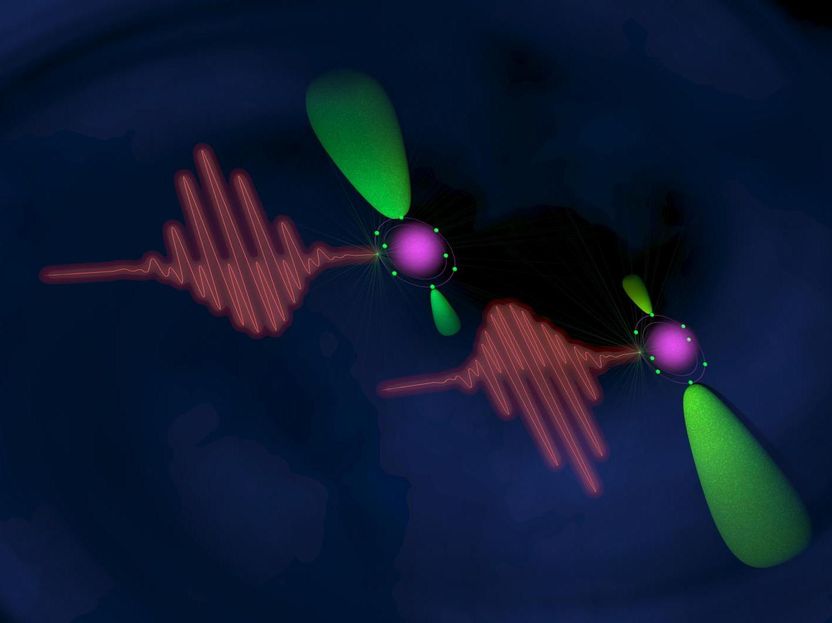
Scheme of the experiment: pulses of light (waves) emit electrons (green) from a neon atom (violet).
Image courtesy of Maurizio Contran, Department of Physics, Politecnico di Milano.
Chemical, physical and biological processes are intrinsically dynamic, because they depend not only on the atomic and electronic structure of matter, but also on how they evolve in time. Ahmed Zewail won the Nobel prize (1999) for "femtochemistry": the observation and control of dynamic chemical processes using ultrafast laser pulses, of a few millionths of a billionth of a second (femtoseconds). This is the scale of time on which atoms make or break bonds in chemical or biological processes, such as photosynthesis or combustion.
Nature however can be still "faster". The atoms in a molecule move on the scale of femtoseconds, but the electrons, which are the basis of chemical bonds, are much faster and in the processes they cause, they move a thousand times faster, that is, tens or hundreds of attoseconds (a billionth of a billionth of a second).
"Like many in the scientific community", explains Kevin Prince, first author of the article just published, "we have also been working for years to develop innovative analytical methods with attosecond resolution to study and control fast dynamics. With this work, that exploits the exceptional properties of the laser light from FERMI, we can say we have finally achieved our goal."
The result was achieved by an international team of researchers from Italy (Elettra-Sincrotrone Trieste, the Politecnico of Milano, the IFN, IOM and ISM institutes of CNR and ENEA), Japan (Tohoku University), Russia (Lomonosov Moscow State University), USA (Drake University, Des Moines, Iowa) and Germany (Technical University of Berlin, University of Freiburg, European XFEL, Hamburg, Max Planck Institute for Nuclear Physics, Heidelberg).
They used a beam of light of two wavelengths (that is, two different colours) and managed to control the direction of emission of electrons ejected from an atom by the light. The experiment had a time resolution of 3 attoseconds, which now makes possible the study and control extremely fast processes.
"This result opens a new avenue to study and control ultrafast processes that involve electron motion on the time scale of attoseconds. We are dreaming about controlling more complex processes such as photocatalytic processes where the charge transfer plays a key role" said Kiyoshi Ueda, who with his group at Tohoku University, contributed to planning and conducting the experiment, and analysing the results.
Original publication
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.