Seeing energized light-active molecules proves quick work for Argonne scientists
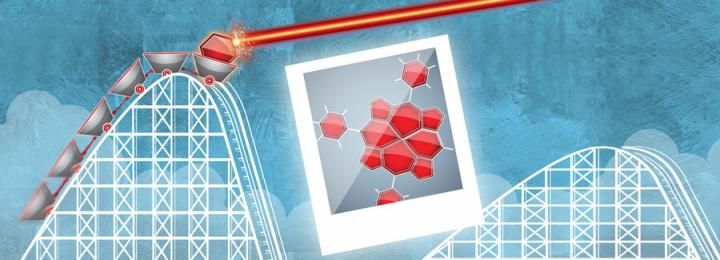
For people who enjoy amusement parks, one of the most thrilling sensations comes at the top of a roller coaster, in the split second between the end of the climb and the rush of the descent. Trying to take a picture at exactly the moment that the roller coaster reaches its zenith can be difficult because the drop happens so suddenly.
To understand how molecules undergo light-driven chemical transformations, scientists need to be able to follow the atoms and electrons within the energized molecule as it gains and loses energy. In a recent study, a team of researchers at Argonne, Northwestern University and the Technical University of Denmark used the ultrafast high-intensity pulsed X-rays produced by the Linac Coherent Light Source to take molecular snapshots of these molecules.
Illustration by Scott Nychay
For chemists trying to take pictures of energized molecules, the dilemma is precisely the same, if not trickier. When certain molecules are excited -- like a roller coaster poised at the very top of its run - they often stay in their new state for only an instant before "falling" into a lower energy state.
To understand how molecules undergo light-driven chemical transformations, scientists need to be able to follow the atoms and electrons within the energized molecule as it rides on the energy "roller coaster."
In a recent study, a team of researchers at the U.S. Department of Energy's (DOE) Argonne National Laboratory, Northwestern University and the Technical University of Denmark used the ultrafast high-intensity pulsed X-rays produced by the Linac Coherent Light Source (LCLS), a DOE Office of Science User Facility at SLAC National Accelerator Laboratory, to take molecular snapshots of these molecules.
By using the LCLS, the researchers were able to capture atomic and electronic arrangements within the molecule that had lifetimes as short as 50 femtoseconds -- which is about the amount of time it takes light to travel the width of a human hair.
"We can see changes in these energized molecules which happen incredibly quickly," said Lin Chen, an Argonne senior chemist and professor of chemistry at Northwestern University who led the research.
Chen and her team looked the structure of a metalloporphyrin, a molecule similar to important building blocks for natural and artificial photosynthesis. Metalloporphyrins are of interest to scientists who seek to convert solar energy into fuel by splitting water to generate hydrogen or converting carbon dioxide into sugars or other types of fuels.
Specifically, the research team examined how the metalloporphyrin changes after it is excited with a laser. They discovered an extremely short-lived "transient state" that lasted only a few hundred femtoseconds before the molecule relaxed into a lower energy state.
"Although we had previously captured the molecular structure of a longer-lived state, the structure of this transient state eluded our detection because its lifetime was too short," Chen said.
When the laser pulse hits the molecule, an electron from the outer ring moves into the nickel metal center. This creates a charge imbalance, which in turn creates an instability within the whole molecule. In short order, another electron from the nickel migrates back to the outer ring, and the excited electron falls back into the lower open orbital to take its place.
"This first state appears and disappears so quickly, but it's imperative for the development of things like solar fuels," Chen said. "Ideally, we want to find ways to make this state last longer to enable the subsequent chemical processes that may lead to catalysis, but just being able to see that it is there in the first place is important."
The challenge, Chen said, is to prolong the lifetime of the excited state through the design of the metalloporphyrin molecule. "From this study, we gained knowledge of which molecular structural element, such as bond length and planarity of the ring, can influence the excited state property," Chen said. "With these results we might be able to design a system to allow us to harvest much of the energy in the excited state."
Original publication
Megan L. Shelby, Patrick J. Lestrange, Nicholas E. Jackson, Kristoffer Haldrup, Michael W. Mara, Andrew B. Stickrath, Diling Zhu, Henrik T. Lemke, Matthieu Chollet, Brian M. Hoffman, Xiaosong Li, and Lin X. Chen; "Ultrafast Excited State Relaxation of a Metalloporphyrin Revealed by Femtosecond X-ray Absorption Spectroscopy"; JACS; 2016
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!