How protein production gets distorted in skin cancer
Each cell in the body follows a strict protocol for manufacturing the proteins it needs to function. When a cell turns cancerous, however, its protein production goes off script. A new study led by researchers at The Rockefeller University takes a close look at one way in which this procedure goes haywire in skin cells as they turn cancerous.
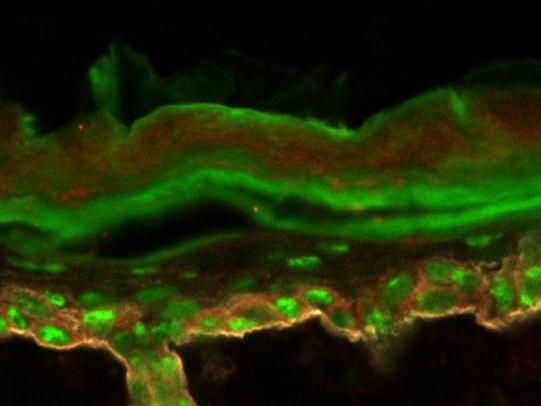
To examine how the production of protein can shift as cells become malignant, the researchers first primed mouse skin to express a molecule (green) common in tumors.
Laboratory of Mammalian Cell Biology at The Rockefeller University/Nature
"A cell's identity depends on the levels of proteins it produces, and these can be altered by changes in the way proteins are translated from genetic instructions," says senior author Elaine Fuchs , the Rebecca C. Lancefield Professor and head of the Robin Chemers Neustein Laboratory of Mammalian Cell Biology and Development .
"Changes in translation appear to be particularly important as normal stem cells become malignant, and our new experiments detail the control mechanisms behind a shift that occurs just prior to the development of skin cancer," adds Fuchs, who is a Howard Hughes Medical Institute investigator. The research describes a potential avenue for future cancer treatments.
In order to function, cells need to turn instructions encoded in their DNA into protein. They do so in two major steps: first, DNA is transcribed into a molecule called messenger RNA, which is then translated into protein. Certain cancerous tumors are known to contain an unusual ratio of protein to messenger RNA, however, which suggests translation is altered in cancer.
Using mice, the team explored changes in translation that occur as the animals develop a common type of skin cancer called squamous cell carcinoma. Adapting a technique developed for cultured cells in co-author Jonathan Weissman's lab at the University of California, San Francisco, they captured messenger RNA as it was being translated within skin stem cells in mice. These molecules were collected from both normal skin stem cells and those primed to become malignant.
Within the pre-malignant cells, the researchers found decreases in regular protein production as well as an uptick in tumor-promoting proteins. Ataman Sendoel, a postdoc in the Fuchs lab and first author on the study, traced this shift back to a change in the proteins that kick off the process of translation. In regular skin cells, eIF2 has this job; in the soon-to-be cancerous cells, he found that eIF2 was inactivated, and its relative, eIF2A, had taken over.
Next, the researchers looked for evidence that eIF2A has a similar role in humans. By searching the publicly available Cancer Genome Atlas, which contains genetic data from 11,000 patients, they found extra copies of the gene encoding eIF2A in 29 percent of patients with squamous cell carcinomas in the lungs, and in 15 percent of those with tumors in the head or neck. Moreover, patients in whose cells the gene was more active had a poorer prognosis, surviving and remaining disease-free for less time compared to those with normal eIF2A activity.
The team's work suggests eIF2A could potentially provide a therapeutic target for new cancer treatments, and the researchers have begun investigating this possibility with help from a grant from the Robertson Therapeutic Development Fund .
"By looking for molecular inhibitors that can turn off eIF2A--and, as a result the translation of cancer-associated proteins--we suspect it may be possible to stop the formation of new tumors," says Sendoel. "For instance, one could envision using such a treatment after tumor surgery to inhibit any tumor-initiating cells that remain."