The stickiness of molecules
What we could not measure before
When building with molecules, it is important to understand how they stick to each other. The problem is that the methods used to measure this are themselves an influencing factor on the process. Researchers at TU Eindhoven, led by Professor Bert Meijer, present a method that excludes this influence and which can measure how fast small molecules detach from a larger molecular entity dissolved in water. What is special about this method is that it is normally used for quite a different application.
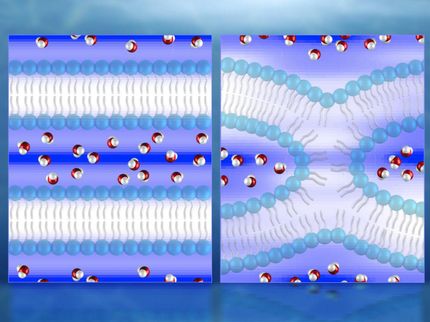
A schematic picture of the new technique. The red dots represent deuterium atoms. The monomers that leave the polymer (depicted left) will be exposed to the deuterium in the heavy water, resulting in the replacement of the hydrogen atom by a deuterium atom that is just a bit heavier.
Eindhoven University of Technology
Before a car mechanic can make a car, he needs to know about the constituent components. The same is true of 'building' with molecules, for example, to create capsules to transport drugs through the human body or to make a medical hydrogel for local drug delivery and stem cell therapy.
Monomers for polymers
Such capsules or materials tend to be made of polymers that, in turn, are constructed of smaller building blocks, or monomers. In self-assembling molecules these monomers form polymers by themselves, for instance in the form of long threads or small pellets in which drugs can be transported.
The monomers in these self-assembling, supramolecular polymers are not attached to each other but lightly stick to each other. This gives the monomers scope to detach from and reattach to the polymer. The ambient temperature or acidity (pH) has an influence on this mobility. So it is important for researchers or manufacturers to know about this if they want to employ capsules in the human body where the temperature and acidity level are not the same everywhere.
Deuterium instead of dye
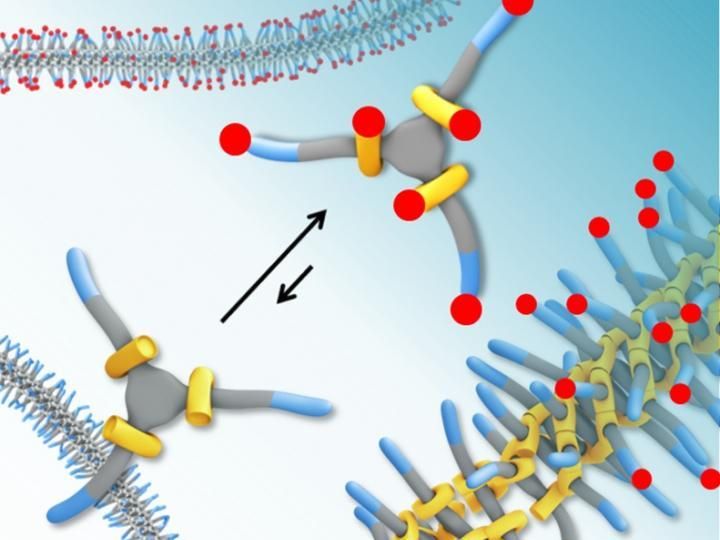
Measuring such mobility is usually done by coupling a dye to the molecule but the problem with this is that the dye is heavier than the molecule and thus has an influence on the movement itself. Doctoral student René Lafleur, together with colleague Xianwen Lou, has now demonstrated that this is not the case when using the 'hydrogen/deuterium exchange mass spectrometry' method. This method is already used to study the folding of proteins - also a type of polymer -- but to date has not been used for this application.
So how does it work? Once the monomers that have been dissolved in water have stuck to each other and formed a polymer, the researchers dissolve them in heavy water. The monomers that detach from the polymer come into contact with the deuterium in the heavy water, whereby the hydrogen atom is replaced by a deuterium atom, which is just that bit heavier. The extra weight, however, is around 450 times smaller than the dye that is currently employed, and so this extra weight does not affect the mobility.
Smaller movements can be measured
The small change in mass can be detected by Lafleur and Lou, and it can be measured once again when the monomer reattaches to the polymer. The speed at which the monomers increase in mass therefore provides a measure for the speed at which the monomers detach from the polymer.
A special aspect of the research results is that while many monomers detach from the polymer within a few minutes and thus increase in mass, others need a period of hours or even days to do so. In addition, the researchers have demonstrated that a small change in the size of the monomer affects the mobility. Larger monomers remain in the polymer for longer and are less quickly mobile than smaller monomers. These differences could not be measured before because that dye molecules employed were too large; the HDX-MS method can now even measure the effect of the small differences in molecule size on the mobility of the molecules.
Original publication
Xianwen Lou, René P. M. Lafleur, Christianus M. A. Leenders, Sandra M. C. Schoenmakers, Nicholas M. Matsumoto, Matthew B. Baker, Joost L. J. van Dongen, Anja R. A. Palmans & E W Meijer; "Dynamic diversity of synthetic supramolecular polymers in water as revealed by hydrogen/deuterium exchange"; Nature Comm.; 2017
These products might interest you
See the theme worlds for related content
Topic World Mass Spectrometry
Mass spectrometry enables us to detect and identify molecules and reveal their structure. Whether in chemistry, biochemistry or forensics - mass spectrometry opens up unexpected insights into the composition of our world. Immerse yourself in the fascinating world of mass spectrometry!

Topic World Mass Spectrometry
Mass spectrometry enables us to detect and identify molecules and reveal their structure. Whether in chemistry, biochemistry or forensics - mass spectrometry opens up unexpected insights into the composition of our world. Immerse yourself in the fascinating world of mass spectrometry!