First Demonstration of Therapeutic Gene Silencing in Primates with Systemic RNAi
Alnylam Pharmaceuticals, Inc. announced the first published demonstration in primates that a systemically delivered RNAi therapeutic can potently silence an endogenous disease-causing gene in a clinically relevant manner. Alnylam and its collaborators at Protiva Biotherapeutics, Inc., demonstrated silencing in primates of the gene for apolipoprotein B (apoB), a protein involved in cholesterol metabolism, with clinically significant efficacy as demonstrated by reductions in levels of cholesterol and low-density lipoproteins (LDL). According to Alnylam, this peer-reviewed research, published in Nature, represents a major advance because it suggests that an RNAi therapeutic can be effective when delivered systemically using a dosage appropriate for application in future human clinical studies.
In the published research, Alnylam scientists and collaborators demonstrated potent silencing in primates of the gene for apoB, a disease-causing protein which to date has not been amenable to targeting with traditional small molecule, protein, or antibody therapies. The achievement of this result by systemic administration through the bloodstream demonstrates the broad potential of RNAi therapeutics to target disease-causing genes, and significantly expands the previously demonstrated opportunity for RNAi therapeutics to treat human disease by direct administration to sites of disease, such as with respiratory or ocular delivery.
In the study Alnylam scientists and collaborators showed that systemic delivery in non-human primates of a chemically optimized small interfering RNA, or siRNA, can result in silencing of the apoB messenger RNA (mRNA), leading to significant reductions in blood levels of the apoB protein. These effects were proven to occur through an RNAi-mediated mechanism, and resulted in immediate, potent, and durable therapeutic efficacy. The siRNAs were formulated with liposomes that enable delivery to liver cells. The observed therapeutic effects included significant reductions in serum levels of cholesterol and LDL, which together represent the so-called "bad cholesterol" associated with development of atherosclerosis and coronary artery disease. Following administration of a single intravenous bolus dose at low dosages from 1.0-2.5 mg/kg, these reductions were observed as early as 24 hours after treatment and lasted for at least 11 days. A single siRNA injection resulted in dose-dependent silencing of apoB mRNA expression, with maximal silencing of over 90%. The silencing of apoB was proven to occur through an RNAi-mediated mechanism of action. In addition, plasma apoB levels were reduced by more than 75%, cholesterol levels by more than 60%, and LDL levels by more than 80%. In the study of 18 animals, the treatment was well tolerated with only transient liver enzyme elevation observed at the highest dose.
Topics
Organizations
Other news from the department research and development

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
NSF and EPA establish 2 centers for environmental implications of nanotechnology - Centers will focus on environmental effects of nanotechnology and its applications
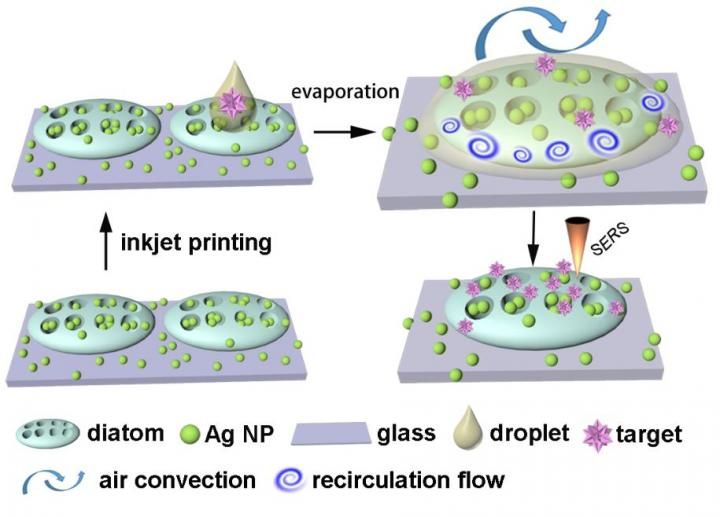
New technology taps power of diatoms to dramatically improve sensor performance