Brookhaven Scientists Take "Snapshots" of Enzyme Action
Results advance understanding of how toxic compounds are eliminated from the body
Scientists at the U.S. Department of Energy's Brookhaven National Laboratory, the New York structural biology Center, and SGX Pharmaceuticals, Inc., have determined the atomic crystal structure and functional mechanism of an enzyme essential for eliminating unwanted, non-nutritional compounds such as drugs, industrial chemicals, and toxic compounds from the body. The detailed mechanism of action will help scientists understand how these compounds are eliminated and what goes wrong in cases where normal metabolism fails. The research are published by the Proceedings of the National Academy of Sciences.
According to Brookhaven biologists Eswaramoorthy Subramaniam, the lead author, and Subramanyam Swaminathan, who led the research, most non-nutritional, foreign substances such as drugs and industrial chemicals are insoluble in water. The body uses two main groups of enzymes - flavin-containing monooxygenases (FMOs) and cytochrome P450s - to convert these compounds to soluble forms that can be easily excreted.
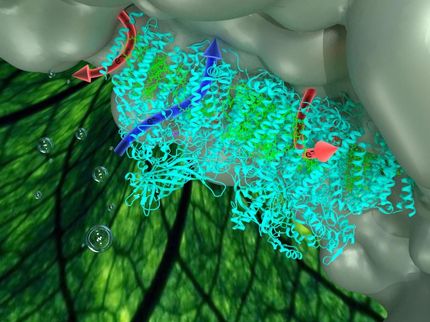
"For FMOs, the end result - that an oxygen atom gets added to make these compounds soluble - is simple," Swaminathan says, "but the reactions require additional participants, or cofactors." In order to understand the molecular mechanism, the scientists used high-intensity x-ray beams at the National Synchrotron Light Source (NSLS) to identify the positions of individual atoms and produce crystal structures of the enzyme, the enzyme plus its cofactor, and the enzyme plus the cofactor plus the compound to be oxidized (the substrate).
"These crystal structures give step-by-step snapshots of different stages of the catalytic action," Swaminathan says, "and reveal a mechanism that is different from what had been known about this process."
Previously, it had been believed that all the "players" - the enzyme, cofactor and substrate - came together at a particular time to perform the function of transferring an oxygen atom from the enzyme to the substrate. "Our finding shows that the substrate and cofactor are binding to the enzyme alternately, not together," Swaminathan says.
First, the cofactor (known as NADPH) binds to a molecule known as FAD, which is a coenzyme attached to the FMO, and transfers a hydride ion to it. That makes the FAD group capable of accepting molecular oxygen. Then, when the substrate arrives, the cofactor leaves so that the substrate can bind to the same site on the FAD group. At this moment an oxygen atom from molecular oxygen is attached to the substrate, and the hydride ion obtained from the cofactor combines with the other oxygen atom to form a water molecule, which is released. Once the substrate is oxygenated, it leaves the enzyme and the cofactor binds again.
"With this back-and-forth, alternating binding, the process repeats over and over for continuous turnover of the product," Swaminathan says.
The details of this process may help scientists understand what happens in cases where compounds are not properly metabolized, and possibly develop corrective measures. One example is a condition called trimethylaminuria, also known as "fish odor syndrome," which results from defective FMOs. Affected individuals are unable to oxygenate trimethylamine, a byproduct of protein digestion released by bacteria living in the gut. People with the disorder release trimethylamine through breath, sweat, and urine, producing a fish-like odor that can be embarrassing and result in psychological effects such as withdrawal and depression.
People with defective FMOs might also suffer additional side effects from drugs, industrial compounds, or other chemicals
Most read news
Topics
Organizations

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.