New filtering technology has environmental, industrial applications
Materials engineers have created a new type of membrane that separates oil from water and, if perfected, might be used for environmental cleanup, water purification and industrial applications. The new technology would last longer than conventional filters for separating oil from water and works by attracting water while beading oil, traits that are usually mutually exclusive. Researchers attached the material to a glass filter commonly used in laboratory research.
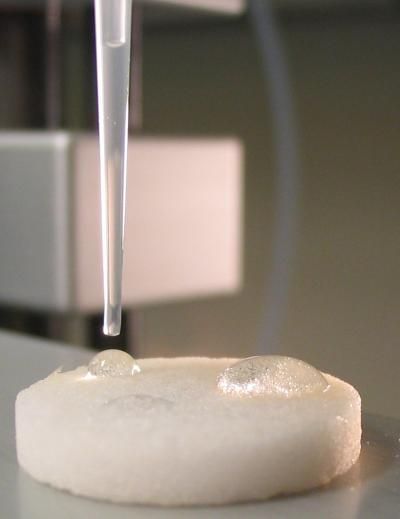
An oily substance called hexadecane beads up on this new type of membrane created by Purdue materials engineers to separate oil from water. If perfected, the new type of filter might be used for environmental cleanup, water purification and industrial applications. The researchers attached their material to a glass filter commonly used in laboratory research, and the modified filter, pictured here, works by attracting water while repelling oil, traits that are usually mutually exclusive.
Purdue School of Materials Engineering
"We take mixtures of oil dispersed in water and run them through these filters, and we are getting 98 percent separation," said Jeffrey Youngblood, an assistant professor of materials engineering at Purdue University. "This is pretty good because if you don't modify the glass filters with our material essentially all the oil goes through. If you modify it with our material, then almost none of the oil goes through."
Findings were detailed in a paper that appeared in the Journal of Colloid and Interface Science, which was written by Youngblood and materials engineering doctoral student John A. Howarte. The findings demonstrate how an oily substance called hexadecane beads up on the membrane while water passes through. The membrane consists of a layer of material called polyethylene glycol, and each molecule is tipped with a Teflon-like "functional group" made with fluorine. Water molecules are attracted to the polyethylene glycol, yet pass through the Teflon-like layer, which acts as a barrier to the oil molecules. The researchers have tested the material with solutions containing oil suspended in water, similar to concentrations existing in oil spills and other environmental cleanup circumstances.
"To clean up an oil spill, for example, you could run contaminated water through a bunch of these filters to remove the oil," Youngblood said. Such filters also might be used in other cleanup applications, such as removing oil from a ship's bilge water or cleaning wastewater contaminated with oil.
The technology might be used in a water-purification technology called reverse osmosis, which now requires a "pre-filter" to remove oil. This "oil coalescence filter" is needed to prevent oil from reaching the reverse osmosis membrane, which is ruined by oil. These conventional oil coalescence filters, however, must be replaced regularly. As water flows through the system, oil sticks to the filters, eventually rendering them ineffective. The new technology, however, would not need to be replaced as frequently because oil does not stick to the filtration material. Instead, the oil droplets could be skimmed off through a commonly used industry technique called cross-flow filtration.
"When the oil-in-water dispersion contacts the filter surface, the oil coalesces to form big droplets. If the mixture is poured onto the filter, the oil forms on top of the material like a layer of cream," Youngblood said.
When a fluid lands on the surface of the material, it forms beads having a distinctive curvature determined by the "contact angle" of the substance. The higher the contact angle, the more a material is likely to form beads. Lowering the contact angle enough prevents substances from beading.
"This material maximizes oil's contact angle while minimizing water's contact angle, allowing water to flow through a filter while holding back the beaded oil," Youngblood said.
A key advantage of the new approach over some conventional methods is that it separates oil from water without using "nanoporous" filters. Filters containing extremely small pores require the water to be pushed through at high pressure, which consumes energy. The new material contains pores between 10 and 174 microns. Because the pores are relatively large, oil-contaminated water would not have to be pumped through.
A patent is pending on the technology.
Topics
Organizations
Other news from the department science
These products might interest you

Microbiology QC products by Cytiva
Efficient membrane filtration for food and beverage testing
Optimize your laboratory tests with versatile filtration systems

FIBRETHERM by C. Gerhardt
Automatic Fibre Extraction for Feed Analysis
FIBRETHERM from C. Gerhardt: Efficient – Precise – Method-Compliant

VICI Jour Katalog 15INT by VICI
The VICI Jour Catalog - Accessories for (U)HPLC and Liquid Handling
Capillaries, Tubing, Fittings, Filters, Safety-Products, Tools and much more

Hahnemühle LifeScience Catalogue Industry & Laboratory by Hahnemühle
Wide variety of Filter Papers for all Laboratory and Industrial Applications
Filtration Solutions in the Life Sciences, Chemical and Pharmaceutical Sectors

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Camouflage mechanism of cancer cells in Hodgkin's lymphoma decoded - Curt Meyer Memorial Prize for Dr. Stephan Mathas and Dr. Martin Janz