To make better MRI images, let the atoms spin out of control
Scientists in the USA and in France have made a new theoretical advance in atomic behavior that could lead to sharper magnetic resonance imaging (MRI) pictures. The discovery could one day help enable the development of portable MRI machines. In the Journal of Chemical Physics, they explain why scientists couldn't completely control the behavior of atomic nuclei during some nuclear magnetic resonance (NMR) experiments.
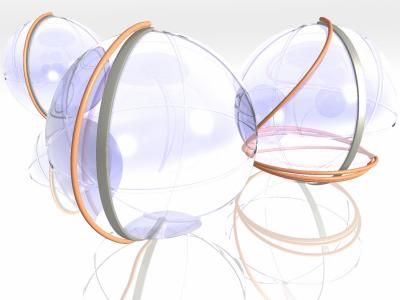
Scientists in Ohio and France have explained some strange atomic behavior, and made a discovery that could ultimately make MRI images sharper. This graphic depicts the quantum mechanical principal of super-adiabaticity, which was responsible for the behavior of atoms in some nuclear magnetic resonance experiments. If the trajectory of the atoms during an experiment were mapped on a globe, then the purpose of an adiabatic experiment is to move the atoms being studied from one point on the globe to another - slowly, and following a very carefully designed path (gray line). With super-adiabaticity, the atoms follow a different - sometimes, wildly different - path (orange line), but still end up at the right destination.
Image courtesy of Philip Grandinetti, Ohio State University.
Some loss of control isn't such a bad thing, explained Philip Grandinetti, professor of chemistry at Ohio State University. Now that he and his colleagues have derived a mathematical explanation of the strange atomic behavior, scientists can use it to make MRI images sharper. The advance may one day help scientists to look inside people and objects without having to put them inside giant magnets - an advance which could lead to portable MRIs.
NMR and its medical counterpart, MRI, reveal the inside of objects by detecting atoms that behave like tiny magnets. Inside the machine's strong magnetic field, atoms align according to north and south magnetic poles, spinning and precessing like tiny tops. Each type of atom broadcasts its identity by emitting a unique radio frequency, depending on its environment.
NMR can reveal the structure of individual molecules. But pictures of complex objects - such as the human brain - often lack detail, because whenever atoms happen to broadcast in opposite directions, they cancel each other out of the final image. In a quest to boost NMR resolution, scientists routinely perform a certain type of experiment that keeps the spins of atoms under very strict control. They refer to such experiments as adiabatic. The Ohio State scientist and his collaborators found that atoms in adiabatic experiments don't always behave as scientists intend them to.
They didn't set out to make such a fundamental discovery, Grandinetti explained. They'd only wanted to examine an earlier University of Alberta experiment which had been aimed at enhancing NMR signal strength.
"We originally wanted to work out a rigorous theoretical description of the Alberta experiment, but the more we tried to understand even the simplest adiabatic process in magnetic resonance, the more we realized that there was a disturbing discrepancy between theory and experiment," he said. They uncovered the same contradiction in many other studies over decades of NMR research. Even though all these experiments seemed to run properly and yield good results, the atoms hadn't spun in a controlled way. There was only the barest mention of the effect in the scientific literature over the years, and nobody had bothered to find the cause.
"To be fair, though, it wasn't clear that this discrepancy posed a real problem, and most people thought the conventional theoretical approach was doing a fine job," he added. "It was only after we fully understood the reason for the discrepancy that we realized the conventional theoretical approach contained a flaw that might prevent better adiabatic processes from being discovered."
The scientists discovered that atoms were not spinning out of control, but rather were moving in a predictable way, according to a quantum mechanical concept called super-adiabaticity. The concept was first proposed in the late 1980s. A super-adiabatic process is still adiabatic. That's why all those seemingly contradictory experiments ran properly - the atoms were behaving in an adiabatic way, just not according to the commonly accepted adiabatic theory.
The idea is based on some very complex mathematics, but it can be visualized in three dimensions. If the trajectory of the atoms during an experiment were mapped on a globe, then the purpose of an adiabatic experiment is to move the atoms being studied from one point on the globe to another - slowly, and following a very carefully designed path.
With super-adiabaticity, the atoms follow a different - sometimes, wildly different - path, but still end up at the right destination. It's as if an airplane was scheduled to fly in a straight line from the North Pole to the South Pole, but instead veered way off course, spiraling southward until it eventually reached the other pole. The route was circuitous, but the end result was the same. In the case of the NMR experiments, super-adiabaticity re-routed the atoms' flight plan.
Grandinetti hopes to incorporate the algorithm into software for controlling NMR and MRI measurements, where it might boost image resolution. One day, it might even help these instruments obtain signals from objects located outside of a magnet. Scientists could also use super-adiabaticity to exert better control over atoms for quantum computing, and to make more precise structural studies of complex biological molecules, he said.