Chemistry for a Raney day
Spanish and Mexican scientists have stumbled across a new way to monoalkylate amines that they claim is more environmentally friendly than alternative routes.
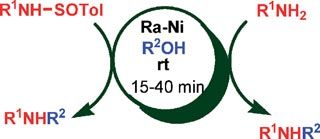
José García Ruano, at the Autonomous University of Madrid, and colleagues were using Raney nickel to cleave nitrogen–sulfur bonds in N-sulfinylamines when they found that changing the solvent produced unexpected products. When they did the reaction in THF, they got primary amines. However, when they changed the solvent to ethanol, they formed N-ethylamines. Although they are not sure of the mechanism, Ruano says he thinks radical intermediates are involved.
Ruano showed that a variety of primary and secondary alcohols can be used to alkylate the amines. Also, he found that dialkylation, a common problem in amine alkylation reactions, does not occur. Ruano recovered the Raney nickel by filtration after the reactions and demonstrated that it could be reused.
‘Many of the methods [for alkylating amines] reported so far are undesirable from an environmental point of view, due to the generation of wasteful byproducts,’ says Ruano. ‘The broad scope of this method, which does not generate byproducts, along with the heterogeneous character of the catalyst suggests it is one of the most convenient methods reported so far.’
Ruano says that the method could be used on an industrial scale because the catalyst is inexpensive and easily recovered. He is now working on monoalkylating amino acids and peptides and also tandem processes where he reduces imines then alkylates them.
Original publication: José Luis García Ruano et al., Chem. Commun., 2009.
Most read news
Topics
Organizations

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.