New tool for structural biology
New method of crystallising large membrane proteins
ETH researchers have developed a new method of crystallising large membrane proteins in order to determine their structure. This will be of benefit to biological research and the pharmaceutical industry.
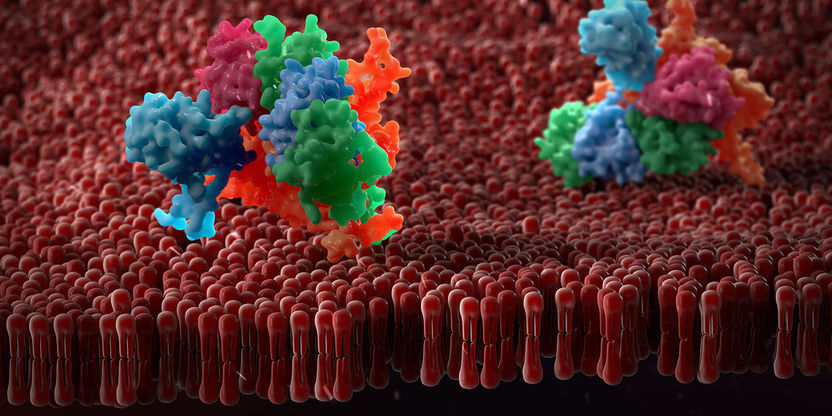
Artistic representation of a membrane with embedded proteins: ETH Zurich researchers have developed a method that will give impetus to the structural elucidation of such molecules.
Copyright: www.colourbox.com
Membrane-embedded proteins are an essential part of cells and any form of life. They not only exist in many different varieties, but also perform a wide range of functions, ranging from intracellular communication and the transport of substances into or out of the cell to mediating the immune response. Membrane proteins are considered important therapeutic and diagnostic target structures. If their structure and functions are known, pharmaceutical researchers can develop active substances that influence these functions in a targeted manner.
Until now, however, elucidating the structure of membrane proteins has been very difficult since it first requires researchers to isolate large numbers of these molecules and form crystals from them. Therein lies the difficulty: membrane proteins are insoluble in water and often too large and heterogeneous to be crystallised using the standard methods.
Now, the group led by Raffaele Mezzenga, Professor of Food and Soft Materials at ETH Zurich, is working to eliminate this restriction. In a publication in the journal Nature Communications, the group presents a general method, which can be used to crystallise membrane proteins of any type or size.
Lipid-water mixture as a reaction chamber
The foundation for the new method was laid in the 1990s with the method called “in meso crystallisation”: the proteins are isolated and concentrated using stable water-lipid mixtures known as lipidic mesophases. In mesophases of this kind, a self-assembly process leads to a three-dimensional network of bent water channels whose walls are made up of lipids, as in a biomembrane. These water channels typically have a diameter of 3-4 nanometres, and the network’s basic cubic motif is repeated at regular intervals.
In channels of this kind, the membrane proteins embed themselves into the walls using the hydrophobic part that otherwise sits in the cell membrane. The rest of the protein ends up in the water channel’s interior, and the proteins, once correctly reconstituted, can then start to crystallise. It is precisely because the channels offer so little space that in the past, only small membrane proteins could be crystallised – large proteins were crushed out and did not form crystals.
Channels expanded using charged lipids
The ETH researchers have now used a trick to expand the channels: they mixed a small proportion of electrically charged lipids in with the lipids. These repel one another and thus inflate the channels, increasing their diameter to 20 nanometres. Although the first attempts to electrostatically swell water channels in lipid mesophases date back to early 2000’s and have continued steadily until recently, this is the first demonstrated evolution of this strategy into a methodology of general significance.
Thanks to these swollen lipidic mesophases, indeed, Mezzenga and his colleagues managed to crystallise large membrane proteins and then elucidate their structure.
The ETH researchers practised on the membrane protein called GLIC (Gloeobacter ligand-gated ion channel), which comes from bacteria. GLIC has several large sub-units that lie outside the bacterial membrane in the outer part of the cell. In the past, a different method was used to crystallise this complex since these domains were too large. “Our procedure not only improved the crystallisation, but also produced extremely compact crystals belonging to a new crystallographic group for this protein,” says Mezzenga. In addition, the researchers were able to crystallise this channel protein in its closed configuration for the first time. Until now, researchers were able to crystallise the complex only in its open state using a different method.
Boost expected for structural elucidation
The new “generalised in meso” method is likely to be of great interest to structural biologists in particular, who until now have struggled to elucidate the structure of large membrane proteins. “This tool will give new impetus to structural elucidation, as it opens up proteins that were previously out of reach,” says Mezzenga.
At present, scientists know the exact structure of only 360 small membrane proteins, or about a seventh of all membrane proteins. The structure of the many remaining membrane proteins is unknown.
According to Mezzenga, the research may also be of benefit to the pharmaceutical industry. “The ability to determine structure is of paramount importance for the development of new drugs,” he says. “This method will make it considerably easier and provide new impetus in the field.”
Successful end to a long project
The development is not covered by a patent. “We purposely published the paper in an open access journal, so that all interested researchers have unlimited access to it,” says Mezzenga, who has invested more than three years of work into this project. He began the initial experiments back in 2015 and then continued in 2016 during a sabbatical at RMIT University in Melbourne; further experiments, such as the structural elucidation of GLIC, were conducted using the synchrotron light source at the Paul Scherrer Institute (PSI) in 2017. The project’s funding included a grant from the Swiss National Science Foundation (SNSF).