On the Track of Nitrogenase
Decisive step towards understanding biological nitrogen fixation taken
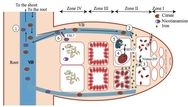
Another step has been taken towards explaining the molecular mechanisms of biological fixation of nitrogen by the enzyme nitrogenase: Dr. Daniel Sippel from Prof. Dr. Oliver Einsle’s work group at the Institute of biochemistry of the University of Freiburg has shown that the iron-vanadium cofactor (FeVco) of the enzyme can release and re-bind one single sulfur ion. Previously, the Freiburg researchers were able to partly analyze the center of another nitrogenase, the iron-molybdenum cofactor (FeMoco). In order to understand its reactions better, Schiller studied a special variant of the enzyme, which contained the element vanadium instead of molybdenum, leading to a series of easily-modified chemical characteristics. When FeVco releases the sulfur ion, the binding site for nitrogen becomes available. So then two amino acids, parts of the protein, are available to supply the protons required for the reaction and keep the permuted nitrogen compound precisely in place.
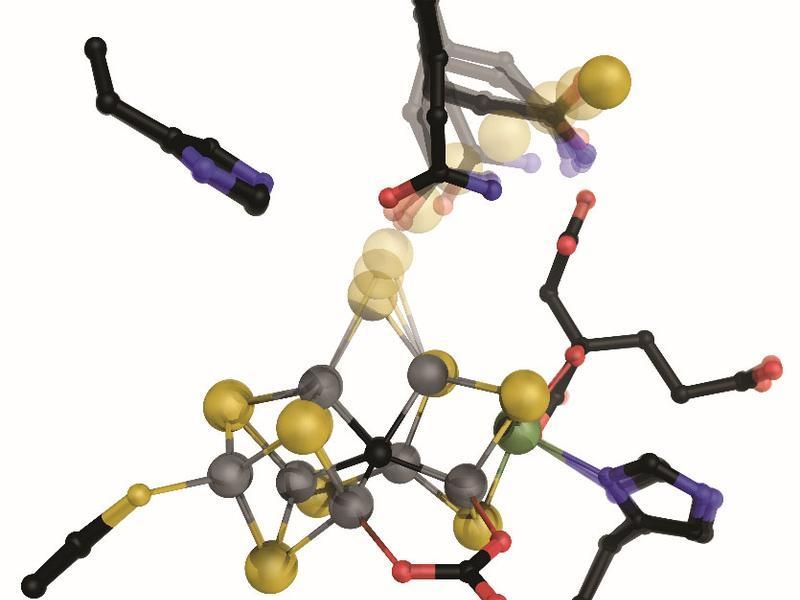
The active center of the vanadium-bearing nitrogenase has a metal center from which a sulfur ion (yellow) is removed after activation, releasing a binding site for nitrogen on two of the seven iron ions (grey).
Oliver Einsle
The work group has been studying nitrogenase for a long time with the aim of making it usable for biotechnology. With it they hope to offer an alternative to industrial chemical fertilization methods such as the Haber-Bosch process, which combines nitrogen with hydrogen to make ammonia. In nature, nitrogenase is the sole enzyme to create the same reaction, however it does so without releasing surplus nitrogen compounds into the environment. Until now however the functioning of this complex, metalliferous enzyme system has not been fully explained. The information obtained by Sippel will form a new basis for the understanding of the molecular mechanisms of nitrogen fixation. This enables the researchers to understand a variety of accumulated data better and integrate them into an overall picture of the reaction of this enzyme.
Original publication
Most read news
Original publication
Sippel, Daniel and Rohde, Michael and Netzer, Julia and Trncik, Christian and Gies, Jakob and Grunau, Katharina and Djurdjevic, Ivana and Decamps, Laure and Andrade, Susana L. A. and Einsle, Oliver; "A bound reaction intermediate sheds light on the mechanism of nitrogenase"; Science; 2018
Topics
Organizations

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.