Chemists design 'miniecosystems' to test drug function
Scripps Research scientists have solved a major problem in chemistry and drug development by using droplet-sized 'miniecosystems' to quickly see if a molecule can function as a potential therapeutic.
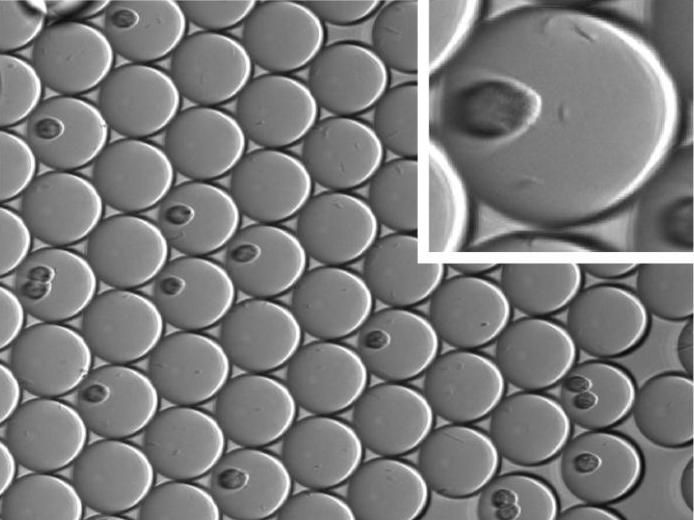
Millions of miniecosystem droplets can be generated quickly for rapidly antibody testing.
Lerner Lab / Scripps Research
As they report the new method will let researchers save critical time and funding by simultaneously testing how drug candidates bind to their cellular targets and alter cell function. The Scripps Research scientists used the technique to assess the therapeutic potential of antibodies, Y-shaped immune system proteins that are the focus of much drug discovery research.
"This could save a lot of time in drug discovery by reducing the steps needed to assess drug candidates," says Tianqing Zheng, PhD, postdoctoral associate on Scripps Research's California campus and first author of the new study.
The study builds on 30 years of research led by study senior author Richard Lerner, MD, Lita Annenberg Hazen Professor of Immunochemistry at Scripps Research, to take advantage of antibody phage display, a technology that scientists can use to label and test antibodies for their ability to bind to a biological target. Antibody phage display technology has propelled the development of pharmaceuticals, from cancer drugs to the blockbuster therapeutic Humira.
But scientists using this method still face a bottleneck: in the vast group of antibodies with a binding affinity for the disease target, there may be only a few antibodies that have the right biological functions. Testing these antibodies for function adds time and expense to the drug discovery process.
The new miniecosystem method tests for affinity and function at the same time. The miniecosystems are held in droplets the size of a picoliter--or one-trillionth of a liter. In these cramped quarters, the researchers brought together a mammalian cell and E. coli bacteria. The bacteria produce phage that serve as carriers for antibody drug candidates. These antibodies on phage surface can interact with the mammalian cell in the same miniecosystem.
"Co-cultivation of mammalian and bacteria cells in mini-ecosystems makes it possible to select functional antibodies directly with phage display," Zheng says.
The mammalian cell in the droplet is engineered to express a fluorescent protein if properly targeted by an antibody. This means that in one step, scientists can test antibody affinity and function, potentially making drug discovery more time- and cost-effective.
To test their new system, the researchers quickly generated millions of miniecosystems with mammalian cells and bacteria that produce phage-tethered antibodies. They tested these antibodies against a real biological target: a receptor on brain cells, called TrkB.
The system worked. On top of that, the researchers were surprised to see that the antibodies did a better job at targeting TrkB when attached to phage, rather than the antibody alone, as they had been in previous studies.
Zheng says the next step is to apply this method to select functional antibodies against many more targets of interest.
Original publication
Most read news
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.