Monitoring the lifecycle of tiny catalyst nanoparticles
Since they are so tiny, it is difficult to investigate single nanoparticles. However, this is exactly what researchers are aiming for in order to tailor their properties. A novel approach: particle at the stick.
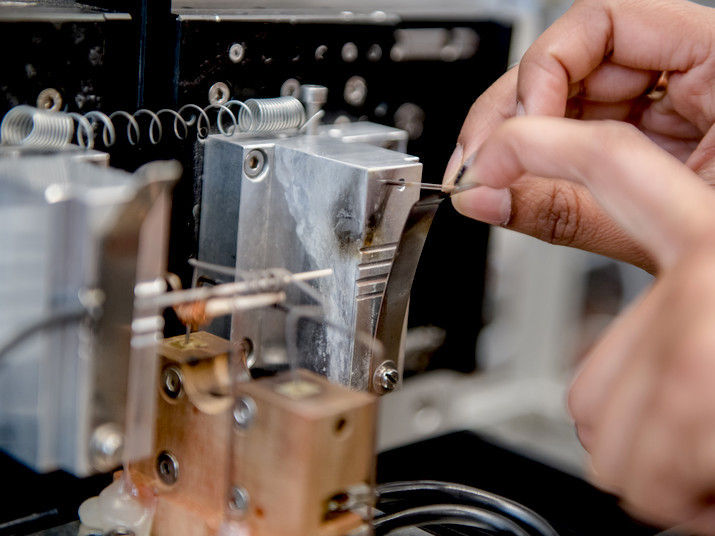
Invisible to the naked eye: at the tip of this carbon nanoelectrode the tiny catalyst particle is located.
© RUB, Marquard
Nanoparticles can be used in many ways as catalysts. To be able to tailor them in such a way that they can catalyse certain reactions selectively and efficiently, researchers need to determine the properties of single particles as precisely as possible. So far, an ensemble of many nanoparticles is analysed. However, the problem of these investigations is that the contributions of different particles interfere, so that the properties of individual particles remain concealed. Researchers at Ruhr-Universiät Bochum in cooperation with colleagues from University of Duisburg-Essen and Technical University of Munich have developed a novel method in order to observe single nanoparticles before, during and after an electrochemical reaction.
Observing the complete lifecycle
“To comprehensively understand the catalytic activity of a nanoparticle, we have to observe how its structure and composition change – from the pre-catalyst to the active catalyst and eventually all the way to the condition after the reaction,“ explains Professor Wolfgang Schuhmann, head of the Center for Electrochemical Sciences. “This is why we have developed the particle at the stick.“
The researchers grew a catalyst nanoparticle at the tip of a carbon nanoelectrode, subsequently activated it and used it to catalyse an electrochemical reaction. Unlike previous approaches, the novel method made it possible for the team to observe the complete lifecycle of the particle.
Fabricating the particle at the stick
In the first step, the chemists modified the carbon nanoelectrode such that the particle preferably attaches to the tip of the electrode. Subsequently, they dipped the electrode’s tip into a solution, which contained the precursor materials for the catalyst. After that, these components assembled automatically, ultimately producing a symmetric particle, in which the constituting elements – the metal cobalt as well as the organic carbonaceous components – were evenly distributed.
The group analysed the shape of the particles by means of transmission electron microscopy. With a special form of X-ray spectroscopy, the researchers determined the elemental distribution within the particle. They repeated these analyses after each step in order to monitor how the particle changed.
Stable nanoassembly of electrode and particle
In the following step, the researchers used heating to trigger the decomposition of the organic compounds and the formation of a carbon matrix with very small embedded cobalt nanoparticles. This is how the actual catalytically active material was formed at the tip of the nanoelectrode.
Afterwards, the chemists used the particle as a catalyst for the production of oxygen from water via electrolysis. The nanoparticle performed excellently and reached turnover rates, which are comparable to industrial electrolysis devices.
“For us it was yet even more important to see that the nanoassembly of electrode and particle was stable enough for a follow-up examination after catalysis,” says Wolfgang Schuhmann. The analysis revealed that the particles underwent considerable restructuring during the reaction. That way, the method makes it possible to monitor the changes of a catalyst at very high turnover rates.
The researchers could not only determine the catalytic activity of an individual nanoparticle with their methodology, but they could also monitor its shape and chemical composition throughout the entire lifecycle – completely without the interference of any other particles.
Original publication
Harshitha Barike Aiyappa, Patrick Wilde, Thomas Quast, Justus Masa, Corina Andronescu, Yen-Ting Chen, Martin Muhler, Roland A. Fischer, Wolfgang Schuhmann; "Oxygen evolution electrocatalysis of a single MOF‐derived composite nanoparticle on the tip of a nanoelectrode"; Angewandte Chemie International Edition; 2019