Direct detection of circulating tumor cells in blood samples
Enhanced Glow
Tumor cells circulating in blood are markers for the early detection and prognosis of cancer. However, detection of these cells is challenging because of their scarcity. In the journal Angewandte Chemie, scientists have now introduced an ultrasensitive method for the direct detection of circulating tumor cells in blood samples. It is based on the amplified, time-resolved fluorescence measurement of luminescent lanthanide ions released from nanoparticles that bind specifically to tumor cells.
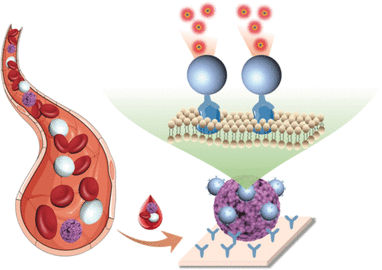
© Wiley-VCH
Conventional techniques for the detection of circulating tumor cells require complicated enrichment before detection because a sample of 10 million blood cells only contains about one tumor cell. In contrast, the new method developed by a team working with Xiaorong Song, Xueyuan Chen, and Zhuo Chen, at Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fujian Agriculture and Forestry University, and Fujian Cancer Hospital (Fuzhou, Fujian, China), works with no enrichment step and directly detects circulating tumor cells in blood samples. The technique is based on a so-called “dissolution-enhanced time-resolved photoluminescence” and uses fluorescing nanoparticles made of lanthanide europium complex.
First the researchers produced antibodies against the epithelial cell adhesion molecule (EpCAM, which is a glycoprotein that is present in very high numbers on the surfaces of many tumor cells and acts as a diagnostic marker for cancer). These antibodies were applied as a coating in the wells of microplates, causing tumor cells contained in the blood sample to remain stuck deep in the wells as other blood components were removed.
The scientists coated the europium-containing nanoparticles with the same antibodies. This caused large numbers of the nanoparticles, added in solution, to specifically bind to the tumor cells. A subsequently added “developer” dissolved the nanoparticles, releasing myriad europium ions. These were immediately bound and tightly locked up by other components of the developer solution. This resulted in a manifold amplification of the fluorescence.
Another essential advantage of this method is that europium ions are very long-lived fluorophores that continue to fluoresce for several microseconds after excitation with a flash of light. Because the measurements are time-resolved, it is possible to start the measurement with a delay. Background signals caused by the autofluorescence of cell components only continue for a few nanoseconds and fade before the measurement begins. This increases the sensitivity of the measurements, making it possible for the researchers to detect a single tumor cell per microplate well.
Tests with blood samples from cancer patients registered as few as 10 cells per milliliter of blood. Fourteen out of fifteen cancer patients were correctly identified by this new method. The number of tumor cells in the samples correlated strongly with the stage of cancer in each patient.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Diagnostics
Diagnostics is at the heart of modern medicine and forms a crucial interface between research and patient care in the biotech and pharmaceutical industries. It not only enables early detection and monitoring of disease, but also plays a central role in individualized medicine by enabling targeted therapies based on an individual's genetic and molecular signature.

Topic world Diagnostics
Diagnostics is at the heart of modern medicine and forms a crucial interface between research and patient care in the biotech and pharmaceutical industries. It not only enables early detection and monitoring of disease, but also plays a central role in individualized medicine by enabling targeted therapies based on an individual's genetic and molecular signature.



![[Fe]-hydrogenase catalysis visualized using para-hydrogen-enhanced nuclear magnetic resonance spectroscopy](https://img.chemie.de/Portal/News/675fd46b9b54f_sBuG8s4sS.png?tr=w-712,h-534,cm-extract,x-0,y-16:n-xl)