Nanoparticles in lithium-sulphur batteries detected with neutron experiment
An HZB team has for the first time precisely analysed how nanoparticles of lithium sulphide and sulphur precipitate onto battery electrodes during the course of the charging cycle. The results can help increase the service life of lithium-sulphur batteries.
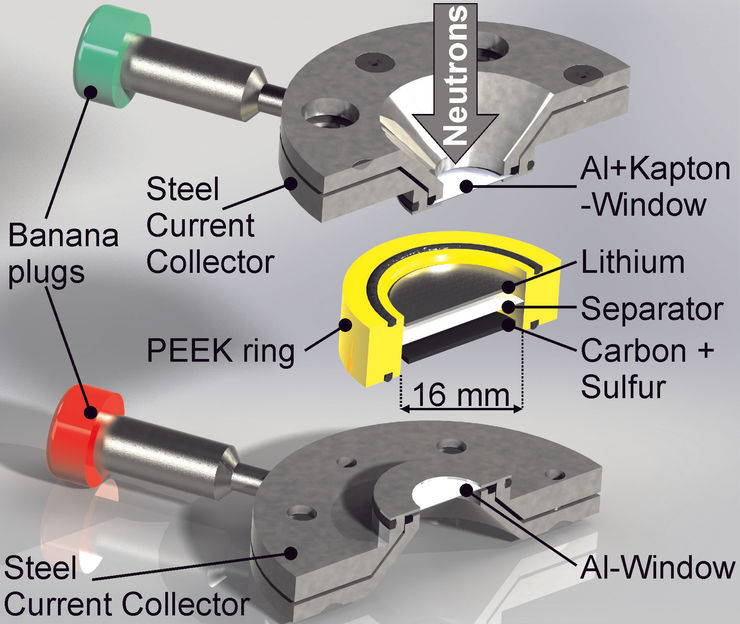
The operando cell was developed at HZB and allows to analyse processes inside the battery during charging cycles with neutrons.
© S. Risse/HZB
Lithium-sulphur batteries are regarded as one of the most promising candidates for the next generation of energy storage devices. They have a theoretical gravimetric energy density that is five times higher than that of the best lithium-ion batteries currently available. And they even work at sub-zero temperatures of down to -50 °C. In addition, sulphur is inexpensive and environmentally friendly.
Capacity loss
However, their capacity so far has fallen sharply with every charge-discharge cycle, so that such batteries are not yet long-lasting. The loss of capacity is caused by complicated reaction processes at the electrodes inside the battery cell. It is therefore particularly important to understand exactly how the charge (sulphur) and discharge (lithium sulphide) products precipitate and dissolve. While sulphur precipitates macroscopically and therefore lends itself to examination by imaging techniques or X-ray diffraction during cycling, lithium sulphide is difficult to detect due to its sub-10-nm particle size.
"Operando" observations with neutrons
Insight into this has now been provided for the first time by investigations with the BER II neutron source at the HZB. Dr. Sebastian Risse used a measuring cell he developed to illuminate lithium-sulphur batteries with neutrons during charging and discharging cycles (operando) and simultaneously performed additional measurements with impedance spectroscopy.
This enabled him and his team to analyse the dissolution and precipitation of lithium sulphide with extreme precision during ten discharge/charging cycles. Since neutrons interact strongly with deuterium (heavy hydrogen), the researchers used a deuterated electrolyte in the battery cell to make both the solid products (sulphur and lithium sulphide) visible.
Surprising insight
Their conclusion: “We observed that the lithium sulphide and sulphur precipitation does not take place inside the microporous carbon electrodes, but instead on the outer surface of the carbon fibres”, says Risse. These results provide a valuable guide for the development of better battery electrodes.
Original publication

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
Cryo-force spectroscopy reveals the mechanical properties of DNA components
Frequency-domain STED microscopy for selective background noise suppression - Novel method suppresses background noise in STED microscopy selectively and effectively, with potential for integration into other dual-beam point-scanning techniques