A New Tool for Cryo-Electron Microscopy
New method that combines cryo-EM with a method otherwise used in materials research
Researchers at Forschungszentrum Jülich and Heinrich Heine University Düsseldorf led by Prof. Dr. Carsten Sachse are using cryo-electron microscopy, or cryo-EM for short, to make biomolecules visible at the atomic level. In a paper now published in the journal Nature Methods, they present a new method that combines cryo-EM with a method otherwise used in materials research. The results are also presented and classified in a Nature Briefing.
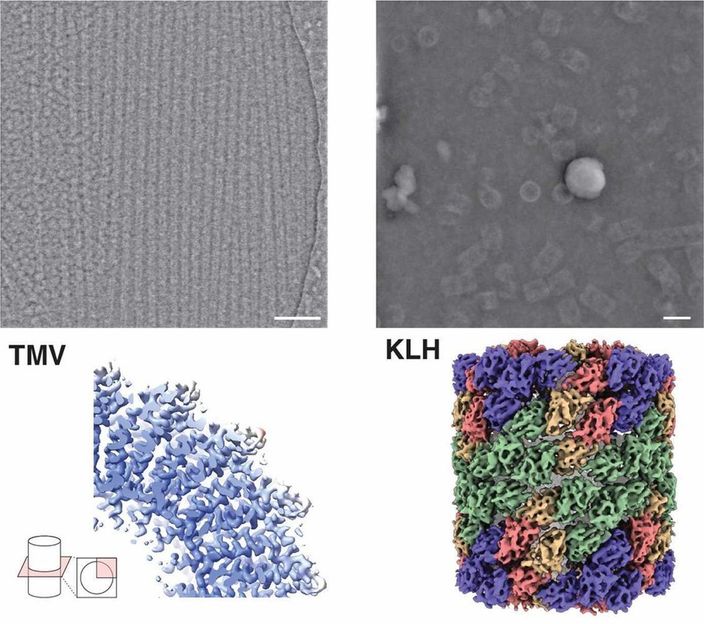
Microscopic image (top) and structure (bottom) of the protein hemocyanin (right) and tobacco mosaic virus (left) by iDPC-STEM. Below the corresponding 3D structures at 3.5 and 6.5 Å resolution.
Forschungszentrum Jülich / Ivan Lazic, Carsten Sachse
The still relatively new technique of cryo-EM has a decisive advantage over X-ray crystallography that has been routinely in use for decades: Protein building blocks can be observed in their natural environment in a snap-frozen state without having to convert them into an artificial crystal beforehand. Cryo-EM is based on transmission electron microscopy. The alternative method that the researchers have now employed, on the other hand, is a further development of scanning transmission electron microscopy with integrated differential phase contrast, or iDPC-STEM for short.
"This method has so far been used primarily in materials research, where it has already led to very high resolutions. When imaging biological samples, we have now directly achieved a quality that was first made possible by cryo-electron microscopy a few years ago," says Carsten Sachse, Director at the Ernst Ruska-Centre of Forschungszentrum Jülich and Professor at Heinrich Heine University Düsseldorf.
Together with research partners from the analytics company Thermo Fischer Scientific in Eindhoven, he was able to map protein structures using iDPC-STEM with a sub-nanometer resolution of 3.5 angstroms. "Cryo-electron microscopy is a bit more advanced today in comparison. But our results show that iDPC-STEM is in principle capable, with some optimization, of achieving similar resolutions to today's cryo-EM and expanding the possibilities for structural analysis; especially for very heterogeneous, non-uniform samples or single particles when averaging capabilities are limited," says Carsten Sachse.
In conventional cryo-electron microscopy, thousands, sometimes tens or hundreds of thousands, of snapshots of a sample are taken from many viewing directions. A powerful computer uses these images to calculate a detailed three-dimensional model of the molecule or particle. Scanning electron microscopy, on the other hand, scans objects line by line in tiny steps to produce a composite image that, as in conventional cryo-EM, serves as the basis for the three-dimensional structure calculation. As with cryo-electron microscopy, a low dose electron beam is used because biomolecules are typically extremely sensitive. This prevents the high energy of the beam from destroying the sensitive structures.
Original publication
Most read news
Original publication
Ivan Lazić, Maarten Wirix, Max Leo Leidl, Felix de Haas, Daniel Mann, Maximilian Beckers, Evgeniya V. Pechnikova, Knut Müller-Caspary, Ricardo Egoavil, Eric G.T. Bosch, Carsten Sachse Single-particle cryo-EM structures from iDPC-STEM at near-atomic resolution Nature Methods (5 September 2022)
Topics
Organizations
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.