New organ-on-chip pilot seeks to reduce animal testing in consumer health industry
Cooperation between Bayer, the start-ups esqLABS and Dynamic42, and Placenta Lab of Jena University Hospital
A first-of-its-kind collaboration in the consumer health industry is developing a platform aimed at reducing or replacing animal testing using “organ-on-chip” (OoC) technology and interactive computational software. The pilot project, supported by esqLABS, Dynamic42, Placenta Lab of Jena University Hospital, and Bayer’s Consumer Health Division aims to generate clinically relevant data, a key step in evaluating new drug candidates in preclinical research.

Symbolic image
Computer-generated image
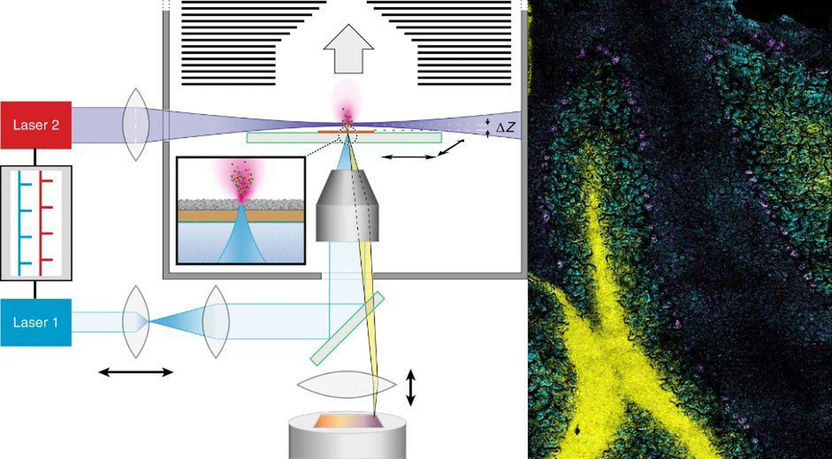
The one-year pilot will focus on evaluating whether small molecules can cross the blood-placenta-barrier in pregnant women, an understudied population due to challenges in conducting clinical research. The platform will consist of a microphysiological system (MPS, so called “organ-on-chip”) representing the main human tissues involved in drug disposition (liver, intestine, placenta) as well as a pumping system to circulate cell culture media among the tissues. The platform will be digitalized to simulate the distribution of compounds and translate the data to human situations.
While animal tests are often required by regulation in the preclinical phase of new drug development, there can be challenges in translating outcomes from animals to humans in some cases. If successful, the platform could help reduce animal testing while improving development outcomes, reducing costs, and last but not least improving patient safety.
Under the terms of the agreement, the companies will bring together esqLABS’ unique expertise in computational modelling of biomedical systems, Dynamic42’s expertise in in-depth tissue and hardware engineering as well as Bayer’s leading expertise in human pharmacokinetic predictions to create the integrated biological and computational platform. The Placenta Lab provides unique experience in developing and building a placenta-on-chip, which is the key element in this study. esqLABS will develop the computational software tool, and Dynamic42 will develop a multi-organ-on-chip platform including the placental barrier. Bayer will provide industry guidance for real-world usage, as well as drug and data sets to help validate the platform’s predictions. Financial details were not disclosed.
Partner quotes
“At esqLABS, we develop software solutions for translational research in the Life Sciences. Our solutions can be leveraged at critical decision points in pre-clinical drug development and the MPS platform that we are developing as a team is a great example integrating virtual twins with bioengineering. We are very excited to work with the scientists from Bayer, Dynamic42, and the Placenta Lab through this collaboration,” said Dr. Christian Maass, OoC-Research Lead at esqLABS. “The consortium shares a common vision and sees an opportunity to design a platform that predicts whether compounds cross the blood-placenta-barrier. This is an unprecedented endeavour and we are very excited to be part of this journey.” adds Dr. Stephan Schaller, CEO of esqLABS.
“Dynamic42 has performed many studies for pharmaceutical purposes in recent years, but this is clearly something new and exciting for us. Challenging the potential of MPS in a multi-organ setting and combining it with in silico predictions is not just a great concept, it perfectly falls in line with new guidelines for pre-clinical drug testing that have been approved recently by the U.S. government in the FDA Modernization Act 2.0. This specifically allows and encourages the use of smart combinations of new technologies and approaches to minimize or replace animal testing. This collaboration between esqLABS, the Placenta Lab of Jena University Hospital, Dynamic42 and Bayer has the potential to lead to a transformational change in how we evaluate human pharmacokinetics, without dosing humans,” said Martin Raasch, CEO of Dynamic42.
“The Placenta Lab is active in a broad spectrum of research topics around human reproduction and pregnancy. Some of our current projects are focused on trophoblast and immunological research, toxicology, and alternatives to animal experiments, since species differences are among the main reasons for the high failure rate of preclinical studies. This collaboration represents a great milestone for the development of new mechanisms for the study of the placenta barrier and its bi-directional selective transport in humans. The strengths of esqLABS, Dynamic42 and Bayer, combined with our experience in the field, work synergistically to offer a path from bench to bedside,” said Priv.-Doz. Dr. Diana Morales, Deputy head and scientist group leader of Placenta Lab.
“Bayer collaborates intensively with stakeholders at national and international levels to develop alternative methods in order to achieve a continuous reduction in the number of animal studies. It has been a major concern of Bayer for years to minimize animal testing according to the 3R principles (Reduce, Refine and Replace). We are thrilled to collaborate with scientists from esqLABS, Dynamic42, and the Placenta Lab through this joint project to find novel ways to minimize animal testing and at the same time seek to generate more reliable and accurate data for product’s safety and efficacy,” said Assoc. Prof. Ramy Ammar, Emerging Science and Innovation Director for Digestive Health at Bayer’s Consumer Health Division.
Something is happening in the life science industry ...
This is what true pioneering spirit looks like: Plenty of innovative start-ups are bringing fresh ideas, lifeblood and entrepreneurial spirit to change tomorrow's world for the better. Immerse yourself in the world of these young companies and take the opportunity to get in touch with the founders.