How cancer genes become independent
Study sheds light on the mysterious evolution of DNA rings
Tumors sometimes seem to take on a life of their own, growing at an unusually fast rate or suddenly developing resistance to a cancer drug. This behavior is often explained by cancer genes separating from the cell’s own chromosomes and “striking out on their own” in ring shapes. So far, little has been known about how exactly these DNA rings arise and how they continue to develop as the tumor grows. An international team of researchers led by Charité – Universitätsmedizin Berlin and the Max Delbrück Center has now harnessed a new method to trace this path in neuroblastoma, a type of cancer. The results have been published in the journal Nature Genetics.
Considered one of the biggest challenges in cancer research, DNA rings are tiny loops of genetic material floating around the nucleus of the cell by the hundreds, detached from the chromosomes. They were first discovered in 1965 and still pose many questions for researchers. Where do all these rings come from? What is their function? How do they affect the cells and the organism as a whole? One thing is clear: Nearly one-third of all tumors in pediatric and adult patients have DNA rings inside their cells – and those tumors are almost always highly aggressive. Ring-shaped DNA, termed extrachromosomal DNA (ecDNA), is also often implicated when a tumor develops resistance to a previously effective medication. Researchers around the world hope to identify new approaches to treating cancer by studying this specific form of genetic information. However, ecDNA does not always have a detrimental effect on cancer growth. Some of the rings also seem to be harmless.
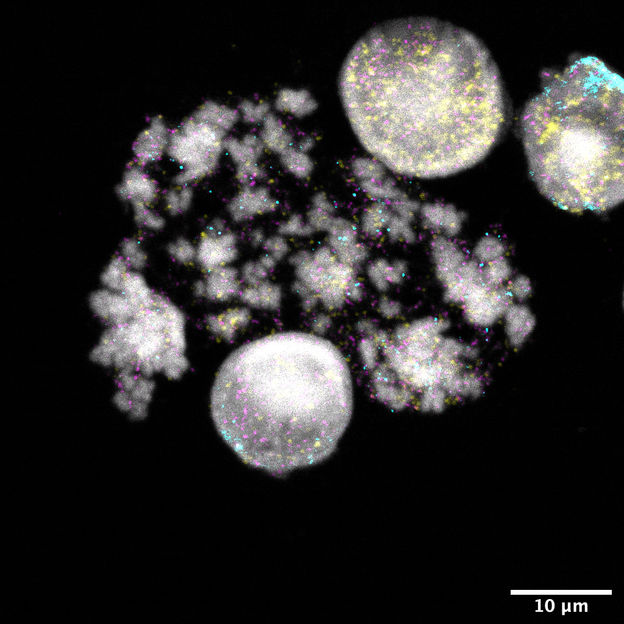
Nuclei and chromosomes of neuroblastoma cells. DNA rings are colored yellow, turquoise, or magenta. Each color is associated with different cancer genes.
Rocío Chamorro González, Charité
“To tell the difference between dangerous and harmless DNA rings and be able to trace their evolution within the tumor, we have to look at the tissue one cell at a time,” explains the head of the study, Prof. Dr. Anton Henssen. He works at the Department of Pediatric Oncology and Hematology at Charité and does research at the Experimental and Clinical Research Center (ECRC), a joint institution of Charité and the Max Delbrück Center. Together with his team, he has now developed a technology that can read the genetic code of the existing DNA rings for each individual cell. At the same time, it tells which genes are active on the rings. “This lets us simply count how many cells in the tumor are home to a specific ring,” Henssen says. “If there aren’t many, then that ring is not highly relevant to the growth of the cancer. But if there are a lot of them, it evidently gives a tumor cell a selective advantage.”
Which DNA rings spur tumor growth?
The researchers initially used the new method to take a snapshot of all DNA rings in cultured neuroblastoma cells. Neuroblastoma is a form of highly malignant cancer that is especially prevalent in very young children. The research showed that no two cancer cells are alike – where one might have 100 DNA rings floating around, the next might have 2,000. The rings also vary greatly in size, with the smallest of them consisting of only 30 genetic components and the largest comprising over a million.
“The big DNA rings are loaded with cancer genes originating in the chromosomes of the cell,” explains Rocío Chamorro González, the study’s first author, who also does research at the Department of Pediatric Oncology and Hematology at Charité and the ECRC. “The ring shape lets them circumvent the classic laws of genetics, so they take on a kind of autonomy. These cancer genes have struck out on their own, if you will. We are only just starting to understand the ramifications. In our study, we found the large DNA rings in many neuroblastoma cells, so they are evidently spurring cell growth. The small rings were only found in isolation, so they are probably not very relevant to the cancer cells.”
Evolution of an independent cancer gene
To understand how an ecDNA originates in the first place and then evolves within a tumor, the second step for the research group was to analyze a pediatric neuroblastoma – cell by cell. Their findings suggest that MYCN, a known cancer gene, first detached from its chromosome of origin and formed a ring shape at the start of the tumor’s growth in this case. Then two of the rings merged to form a larger one, which went on to lose a shorter segment and then a longer one. “The last ring seems to have been the first to confer a growth advantage, because it is the only one that appears in many of the neuroblastoma cells,” Henssen says. “This shows that the cancer gene not only became independent through these processes, but also continued to ‘improve.’”
This kind of insight into the evolution of DNA rings within a tumor would have been impossible if not for the newly developed method. The team of researchers now plans to use the same method to reconstruct the stages of development in further cases of cancer. The researchers hope this will allow them to distinguish better between dangerous and harmless DNA rings. “Our hope is that in the future, we will be able to see in an individual case whether or not that tumor is especially aggressive, just from looking at the DNA rings,” Henssen says. “And then we could adjust the treatment. That’s why testing the predictive power of specific DNA rings is the next target for our research.”