Design organoids with light
Organoids help researchers understand biological processes in health and disease
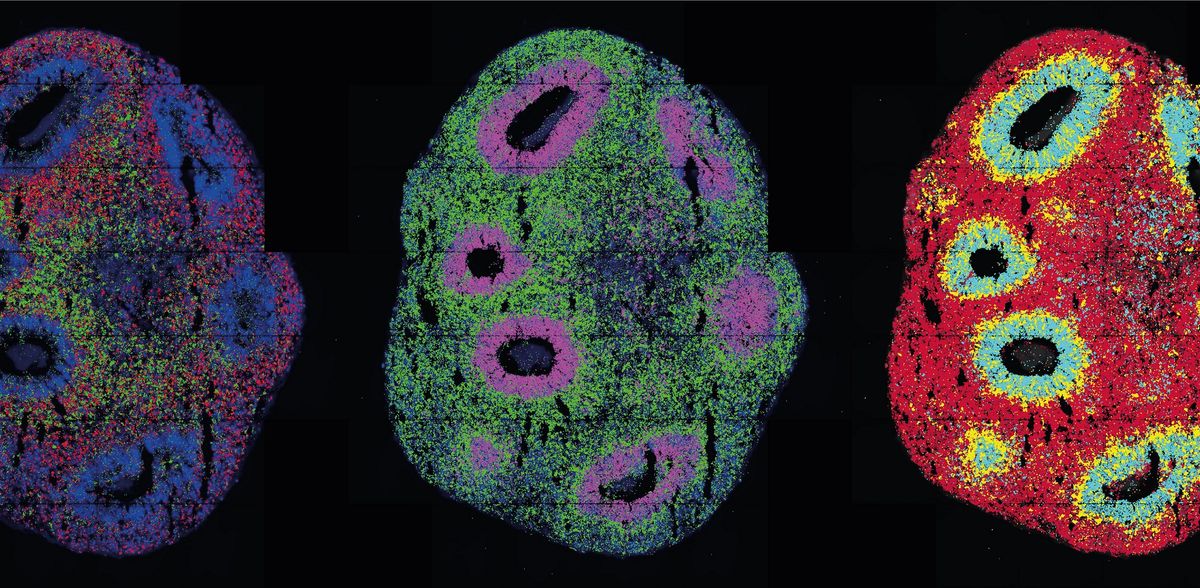
They look like thunderclouds in pinhead format: organoids. The three-dimensional cell cultures play an important role in medical and clinical research because they reproduce tissue structures and organ functions in the Petri dish. Scientists can use them to understand how diseases arise, organs develop or drugs work. With the help of single-cell technologies, they can penetrate to the molecular level of the cells. Thanks to spatial transcriptomics, they can even observe in 3D which genes are active at which location in the organoid over time.
The "miniature organs" are usually developed from stem cells. These are cells that are not yet or barely differentiated. They can develop into any cell type, such as heart or kidney cells, muscle cells or neurons. Scientists "feed" them with growth factors and embed them in a nutrient solution. There they form tiny cell clumps in which they finally function and interact as if they were in real tissue. Until now, it has hardly been possible to control this process. Researchers led by Professor Nikolaus Rajewsky, director of the Berlin Institute for Medical Systems Biology at the Max Delbrück Center (MDC-BIMSB), now describe in the journal Nature Methods a technology that allows them to trigger it, control it and observe it in spatial and temporal resolution. "To do this, we have combined spatial transcriptomics with optogenetics," says first author Dr. Ivano Legnini. "This enables us not only to control gene expression in living cells, but also to observe its progression."
Light sensors activate or block genes
In optogenetics, natural or engineered "light sensors" are inserted into cells. When light falls on the sensors, they activate or block genes in the cells - depending on how they are programmed. Legnini incorporated such light sensors into neuron precursor cells developed from stem cells to form neural organoids. He did this in collaboration with the Organoids Technology Platform team, led by Agnieszka Rybak-Wolf, Ph.D., and Dr. Robert Patrick Zinzen's Systems Biology of Neuronal Cell and Tissue Differentiation group. The researchers wanted to understand how the nervous system develops in the human embryo. Morphogens play a key role in this process - molecules that signal to neuronal progenitor cells whether they should become neurons in the anterior part of the brain or in the posterior part of the spinal cord, for example. The combination of these molecules creates typical patterns of gene expression during development.
Using light, the researchers activated one of these morphogens, Sonic-Hedgehog (SHH). Subsequent spatially resolved single-cell analyses showed that the cells then arranged themselves into typically patterned organoids. The researchers generated the light pulse in two ways: using either a laser microscope or a digital micromirror microscope that Rajewsky's group developed with Dr. Andrew Woehler. At the time, Andrew Woehler headed the Max Delbrück Center's light microscopy platform; as of November 2022, he heads the Experimental Technologies Department at the Howard Hughes Medical Institute in Ashburn, USA. A chip with several hundred thousand tiny mirrors is inserted into this special microscope. These can be programmed so that the microscope can create complex patterns of illumination on a sample - unlike with a laser, which hits only a single spot at a time.
Precise - with potential for improvement
"With our method, we can reproduce processes related to gene expression in tissues very precisely in the Petri dish," says Ivano Legnini. Since March of this year, he has been setting up his own research group at the Human Technopole in Milan. Among other things, he wants to improve the spatial and temporal resolution of the technology there and make it applicable to other organoids.
Nikolaus Rajewsky also wants to continue refining the method: "I am very much looking forward to collaborating with optogenetics experts to further improve the technology and apply it to clinically relevant human organoid models."
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.