Two conductors of a chemical reaction
Watching the reaction live
For the first time, researchers at TU Wien have successfully observed the operating principle of so-called promoters in a catalytic reaction in real-time. These promoters play an important role in technology, but so far there is only limited understanding of how they work.
Water molecules in front of the nanoparticle
TU Wien
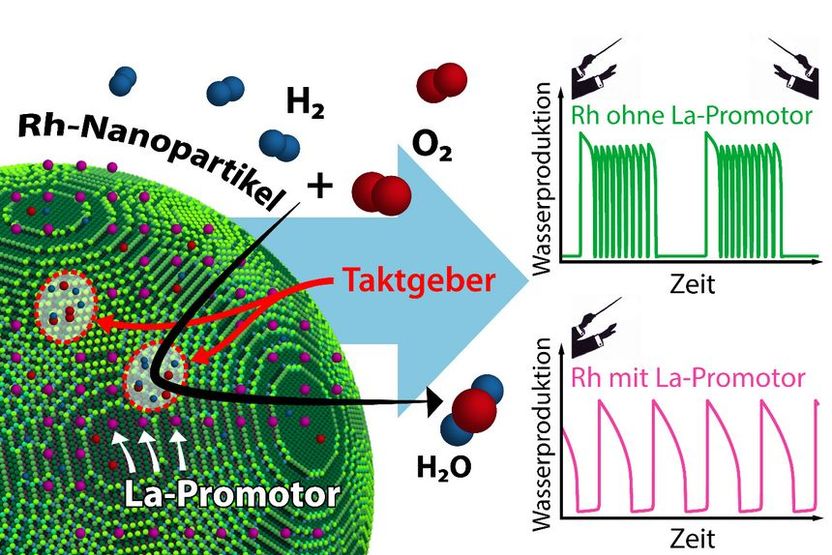
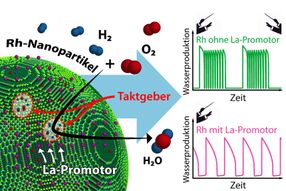
The reaction behavior of an individual nanoparticle is determined by its pacemakers. Addition of a La promoter significantly influences the interaction of these pacemakers.
TU Wien
Catalysts are essential for numerous chemical technologies, ranging from exhaust gas purification to the production of valuable chemicals and energy carriers. Often, tiny traces of additional substances are used alongside catalysts to make them highly effective. These substances are referred to as "promoters." While playing a crucial role in technology, they have been notoriously difficult to study.
In most cases, determining which quantity of promoters has what effects on a catalyst has been a trial-and-error process. However, researchers at TU Wien have managed to directly observe the role of lanthanum promoters in hydrogen oxidation. Using high-tech microscopy methods, they visualized the role of individual La atoms. Their study revealed that two surface areas of the catalyst act as pacemakers, similar to conductors in an orchestra. The promoter plays a vital role in their interaction, controlling the pacemakers. The results of this study have now been published in the journal "Nature Communications."
Watching the Reaction Live
"Many chemical processes use catalysts in the form of tiny nanoparticles," says Prof. Günther Rupprechter from the Institute of Materials Chemistry at TU Wien. While the performance of catalysts can be easily determined through the analysis of products, microscopic insights cannot be gained following this approach.
This has changed now. Over several years, Günther Rupprechter and his team have developed sophisticated methods that allow directly observing individual nanoparticles during a chemical reaction. This enables to see how the activity changes at different locations on these nanoparticles during the course of the reaction.
"We use rhodium nanotips that behave like nanoparticles," says Günther Rupprechter. "They can serve as catalysts, for example, when hydrogen and oxygen are combined to form water molecules – the reaction we are examining in detail."
Oscillating Between "Active" and "Inactive"
In recent years, the TU Wien team already demonstrated that different regions of nanoparticle surfaces exhibit different behaviors: they oscillate between an active and an inactive state. Sometimes, the desired chemical reaction occurs at certain locations, while at other times, it does not.
Using dedicated microscopes, it has been shown that various such oscillations occur on each nanoparticle in parallel, and they all influence each other. Certain regions of the nanoparticle surface, often only a few atom diameters wide, play a more significant role than others: they act as highly efficient "pacemakers," even controlling the chemical oscillations of other regions.
Promoters can now interfere in this pacemaker behavior, and that is precisely what the methods developed at TU Wien have allowed researchers to investigate. When rhodium is used as catalyst, lanthanum can serve as promoter for catalytic reactions. Individual lanthanum atoms were placed on the tiny surface of a rhodium nanoparticle. The same particle was investigated both in the presence and absence of the promoter. This approach revealed in detail the specific effect of individual lanthanum atoms on the progress of the chemical reaction.
Lanthanum Changes Everything
Maximilian Raab, Johannes Zeininger and Carla Weigl have performed the experiments. "The difference is enormous," says Maximilian Raab. "A lanthanum atom can bind oxygen, and that changes the dynamics of the catalytic reaction." The tiny amount of lanthanum alters the coupling between different areas of the nanoparticle.
"Lanthanum can selectively deactivate certain pacemakers," explains Johannes Zeininger. "Imagine an orchestra with two conductors – we would hear quite complex music. The promoter ensures that there is only one pacemaker left, making the situation simpler and more ordered."
In addition to the measurements, the team, supported by Alexander Genest and Yuri Suchorski, developed a mathematical model to simulate the coupling between the nanoparticle's individual areas. This approach offers a more powerful way to describe chemical catalysis than before: not only based on input and output, but in a complex model that considers how different areas of the catalyst switch between activity and inactivity and, controlled by promoters, mutually influence each other.