New hope in the fight against RSV
Researchers discover promising drug candidate
The respiratory syncytial virus (RSV) causes severe lower respiratory tract infections, particularly in young children. To date, there is neither an antiviral therapy against the virus nor a vaccination for children. This is why researchers led by Thomas Pietschmann at TWINCORE, a joint institution of Hannover Medical School (MHH) and the Helmholtz Centre for Infection Research (HZI) in Braunschweig, are looking for new active substances against RSV. In a large-scale study, they have now identified lonafarnib as a promising candidate. They have published their results in the journal Nature Communications.
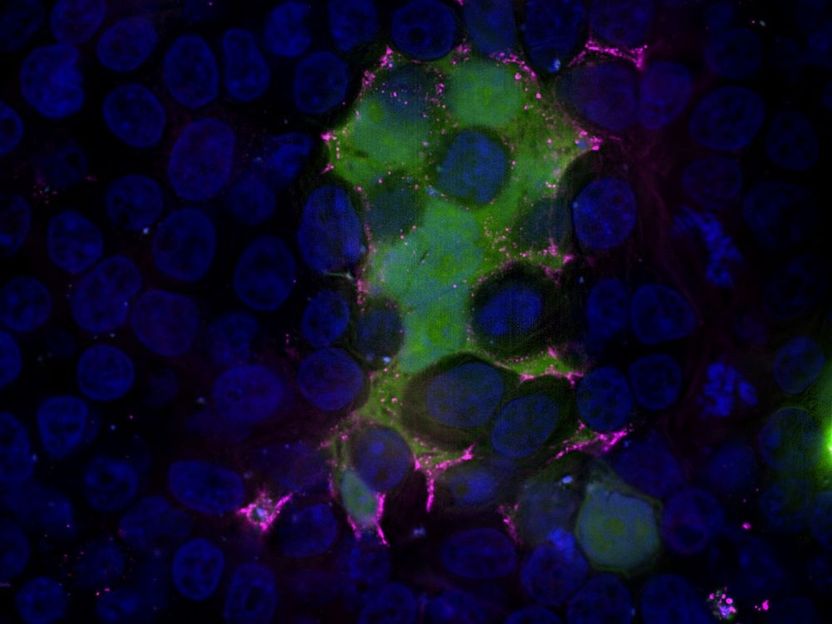
Microscopic image of cells infected with RSV. Green: RSV-F protein labelled with GFP in the cytoplasm of the cells, magenta: RSV-F protein, blue: cell nuclei.
©TWINCORE/Carpentier
Every year in the winter months, there are waves of infection with RSV. In healthy adults and adolescents, the infection is usually harmless. Not so with small children: Around 1% of them who are exposed to the pathogen for the first time become so seriously ill that they have to be hospitalised. It can also cause serious illness in adults over the age of 65 due to pre-existing heart or lung conditions. Vaccines have been authorised for older people and pregnant women since 2023, but there is currently no directly antiviral therapy against the RS virus.
In order to discover new active substances against certain pathogens, researchers search through large collections of already known and clinically tested substances. This process is known as a "drug repurposing screen" and examines additional areas of application for already known pharmaceuticals. The team from the Institute of Experimental Virology at TWINCORE, Center for Experimental and Clinical Infection Research, in Hanover, led by Thomas Pietschmann, used this method to search the ReFRAME Library of the Scripps Research Institute (USA) for potential new RSV drugs. This substance bank contains around 12,000 active substances that are in clinical development or have already been approved.
"To screen the library, we used a so-called reporter virus that is labelled with the fluorescent protein GFP," says Pietschmann. "A lack of fluorescence reaction in this test indicates an antiviral effect." At the same time, all substances were also analysed for their toxicity. Only those that do not have a cell-damaging effect are shortlisted. The tests were carried out automatically using a pipetting robot in collaboration with the Institute of Virology at Hannover Medical School. " Otherwise, it is almost impossible to sift through a collection of several thousand substances," says Sibylle Haid, scientist at the Institute of Experimental Virology and co-corresponding author of the study.
From an initial 21 remaining candidates, the scientists focussed on the active substance lonafarnib, which is approved for the treatment of Hutchinson-Gilford progeria syndrome. People affected by this rare, genetic disease age prematurely and die earlier, on average at 14.5 years of age. "Lonafarnib inhibits a specific maturation step of proteins in the cell," says Haid, "farnesylation." In order to characterise the mechanism of action against the RS virus more precisely, the researchers tested another farnesylation inhibitor called tipifarnib and compared the results. "Tipifarnib does not work against RSV," says Haid. "From this we were able to conclude that the antiviral effect of lonafarnib is probably not based on the inhibition of farnesylation."
With the help of cooperation partners Anna Hirsch from the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) and Thomas Krey from the University of Lübeck, the team was able to elucidate the molecular structure of the virus-drug complex. Lonafarnib binds to the fusion protein of RSV and thus prevents the virus from fusing with the membrane of the target cell. As a result, no new cells can be infected. In cooperation with colleagues in France, a reduction in viral load has already been demonstrated in the mouse model. "However, the dose of lonafarnib required for oral administration is very high, so we have also observed side effects," says Pietschmann. "It is conceivable that local application, for example by inhalation, could improve the ratio between effect and side effect. This must be carefully examined in follow-up studies."
"With lonafarnib, we have identified an interesting candidate for the treatment of RSV," says Svenja Sake, first author of the study. "Because the drug has already undergone all clinical trials, approval for the new indication would be much easier, cheaper and faster than for a completely new active substance," she summarises the advantages of drug repurposing. "This study is also another great example of teamwork, as is usual in science," says study leader Pietschmann. "We are networked with many of the cooperation partners in the RESIST Cluster of Excellence, for example."