Drug discovery within the patient
Genetic analysis of bacteria from humans and animals paves the way for new active ingredients
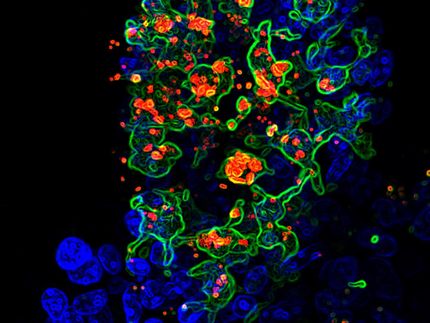
microorganisms do not just colonize the body of mammals during infections. Billions of microbes can be found on and in healthy humans and animals at any given time, communicating with each other via chemical signals and thus influencing their health. In two studies, researchers from the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), Saarland University and Saarland University Hospital have now conducted a detailed study of the microbiome, i.e. the totality of all microorganisms, in humans and zoo animals. The aim was to identify starting points for strategies for the treatment and diagnosis of diseases. The researchers published their results in two articles in the journal Nature Communications. The HIPS is a site of the Helmholtz Centre for Infection Research (HZI) in collaboration with Saarland University.
The idea of using microorganisms as a source of new active ingredients is not new. Numerous drugs have already been developed on the basis of natural products from bacteria and fungi. These compete for available resources in their natural habitat, such as the soil, and use chemical signals to gain an advantage over their microbial competitors. It is therefore not surprising that a large proportion of the antibiotics available on the market is based on natural products from microorganisms. An interdisciplinary research team at the HIPS, Saarland University and Saarland University Hospital has set itself the goal of also finding natural products that can be used to treat non-infectious diseases. Instead of looking in the soil, the team searches directly in the bacteria that colonize humans and animals and play a role in the development of diseases. This approach is also a central component of the planned nextAID³ cluster of excellence, which was positively evaluated in a first round by the German Research Foundation (DFG) and whose full proposal was submitted by Saarland University in August 2024.
The basis for this large-scale search for new active ingredients were two cohorts: In the “IMAGINE” study, the researchers collected almost 2,000 samples from healthy subjects and patients with various diseases. Since different parts of the microbiome can be affected in different diseases, the researchers examined the microbiome in saliva, dental plaque and stool, as well as in the eye, throat and on the skin. In a second study, the team examined the microbiome of animals at Saarbrücken Zoo and compared it to the microbiome of animals living in the wild. The aim of this much smaller cohort was to show that the microbiome of animals can also be a potential source of new natural products. The samples collected in both studies were processed and subjected to a process known as metagenome sequencing. This method allows all the organisms contained in a sample to be genetically identified and their abundance to be determined. If a certain bacterial strain is more or less abundant in the presence of a particular disease, for example, it could potentially be involved in its development or progression.
In the analyzed data, the team, which included bioinformatician Andreas Keller, microbiologist Sören Becker, pulmonologist Robert Bals and pharmacist Rolf Müller, was able to find numerous bacteria for which such a behavior applies. In addition, the researchers were able to use bioinformatics analyses to identify the genetic blueprints (so-called biosynthesis gene clusters) of natural products associated with the diseases under investigation. “To us, this data is a scientific goldmine. We still have no idea which natural products most of the newly identified gene clusters encode,” says Andreas Keller, head of department at the HIPS and Professor of Clinical Bioinformatics at Saarland University. ”The data collected is processed in our recently established database ABC-HuMi. This database already contains further data on the human microbiota, which will allow us to discover many new natural products and use them as a basis for drug development.”
The pharmacists, biologists and chemists at the HIPS are now called upon to use the digital data to create real natural products. A total of six research groups are currently working on the experimental validation of the 50 most promising gene clusters. “Knowing the genetic blueprint of a natural product is only the first step. We are now working hard to transfer the blueprints into bacteria that will use them to produce the encoded natural products,” says University Professor Rolf Müller, head of department and scientific director at the HIPS, who co-initiated the two published studies. ”The data obtained reflect an enormous biodiversity that has not been studied so far. Our next step will be to harness this potential. We are pleased that we can now effectively apply the technologies we have established over the past 15 years for the development of antibiotics to other indications as well.”
While the research focus at HIPS is on antimicrobial agents, therapeutics for non-infectious diseases will be developed as part of the PharmaScienceHub. The collaboration platform established between HIPS and Saarland University in 2023 brings together players from academic research and the pharmaceutical industry to accelerate the translation of findings from basic research into medical applications.
Original publication
Georges P. Schmartz, Jacqueline Rehner, Miriam J. Schuff, Leidy-Alejandra G. Molano, Sören L. Becker, Marcin Krawczyk, Azat Tagirdzhanov, Alexey Gurevich, Richard Francke, Rolf Müller, Verena Keller, Andreas Keller; "Exploring microbial diversity and biosynthetic potential in zoo and wildlife animal microbiomes"; Nature Communications, Volume 15, 2024-9-26