Fuel cells in operation: A closer look
Measuring a fuel cell's overall performance is relatively easy, but measuring its components individually as they work together is a challenge. That's because one of the best experimental techniques for investigating the details of an electrochemical device while it's operating is x-ray photoelectron spectroscopy (XPS). Traditional XPS works only in a vacuum, while fuel cells need gases under pressure to function.
Now a team of scientists from the University of Maryland, the U.S. Department of Energy's Sandia National Laboratories, and DOE's Lawrence Berkeley National Laboratory has used a new kind of XPS, called ambient-pressure XPS (APXPS), to examine every feature of a working solid oxide electrochemical cell. The tests were made while the sample cell operated in an atmosphere of hydrogen and water vapor at one millibar pressure (about one-thousandth atmospheric pressure) and at very high temperatures, up to 750° Celsius (1,382 degrees Fahrenheit).
"Our team, led by Bryan Eichhorn of the Department of Chemistry and Biochemistry at the University of Maryland, combined the expertise in fuel cells at U Maryland, the experience of our Sandia Lab colleagues in collecting electrochemical data, and Berkeley Lab's own development of a method for doing x-ray photoelectron spectroscopy in situ," says Zahid Hussain of Berkeley Lab's Advanced Light Source (ALS). "Together we were able to measure the fundamental properties of a solid oxide fuel cell under realistic operating conditions."
The researchers report their results in the November, 2010 issue of Nature Materials.
How a solid oxide fuel cell works
Like a battery, a fuel cell is a device that uses chemical reactions to produce electricity. Unlike a battery, a fuel cell won't run down as long as it's supplied with fuel and oxidant from outside. The main components are two electrodes, an anode and a cathode, separated by an electrolyte.
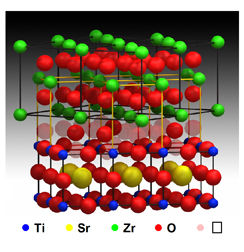
In a solid oxide cell (SOC) the cycle begins at the cathode, which ionizes oxygen (usually from air) by adding free electrons. These oxygen ions then flow through the solid oxide electrolyte (from which the SOC gets its name), often a material known as yttria-stabilized zirconia. High temperature is needed to maintain good conduction of oxygen ions through the electrolyte.
The oxygen ions travel through the electrolyte to reach the anode, where they oxidize the fuel. (The fuel may be pure hydrogen gas or a hydrocarbon.) Electrons freed by oxidation form the current in the device's electrical circuit and eventually return to the cathode. Unused fuel or other compounds, plus water formed from the positive hydrogen ions and negative oxygen ions, exits the fuel cell.
For the APXPS experiment, the University of Maryland collaborators built a model fuel cell that combined the essential elements of an SOC in a special miniaturized design less than two millimeters in length. Except for the electrolyte of yttria-stabilized zirconia, which formed the base of the device, the various components were thin films measuring from 30 nanometers (billionths of a meter) up to 300 nanometers thick.
Says the University of Maryland's Eichhorn, "We designed and fabricated solid oxide electrochemical cells that provided precise dimensional control of all the components, while providing full optical access to the entire cell from anode to cathode."
Instead of stacking the components as in a real fuel cell, the sample's arrangement was a planar design that placed all the components on the same side of the electrolyte, so the x-ray beam from the ALS could reach them. This allowed direct measurement of local chemical states and electric potentials at surfaces and interfaces during the cell's operation.
Introducing ambient-pressure x-ray photoelectron spectroscopy
Photoemission occurs when light ejects electrons from a material. By collecting the emitted electrons and analyzing their energies and trajectories, photoelectron spectroscopy establishes exactly what elements are in the material and their chemical and electronic states within narrow regions. At the Advanced Light Source, intense x-ray light is used to explore what happens at or near the surface of materials: the only photoelectrons that can escape are from atoms near the surface.
The APXPS system begins by shining the x-ray beam on the sample fuel cell inside a chamber at the ambient pressure of the gas needed for it to operate. The emitted electrons then travel through chambers pumped to lower pressure, finally entering the high-vacuum chamber of the detector. By itself this arrangement would lose emitted electrons at every stage because of their spreading trajectories, leaving a signal too weak to be useful. So Berkeley Lab researchers developed a system of "lenses" – not made of glass but of electric fields – to capture and refocus the emitted electrons at each stage, preventing excessive loss.
"This is what allows us to find out what's happening within small regions on the surface of a sample in the presence of a gas," says Hendrik Bluhm of Berkeley Lab's Chemical Sciences Division, one of the inventors of APXPS, which was awarded a coveted R&D 100 Award in 2010. "Using the APXPS instruments at the ALS's molecular environmental science beamline, 11.0.2., and the chemical and materials science beamline, 9.3.2, we can spatially correlate the catalytic activity with the electrical electrical potentials across the different components of the model fuel cells."
Says Zhi Liu of the ALS, "At first we weren't sure we could use this technique with an operating fuel cell, because we had to bring it to 750° C—an extreme temperature for such ambient pressure experiments. Few people have done it before. Now we're able to perform this kind of analysis routinely."
Michael Grass of the ALS says, "What you need to know to improve any kind of fuel cell is where the inefficiencies are—places where energy is being lost compared to what theoretically should be possible. By scanning across the surface of the cell while it was operating, we could directly measure both the inefficiencies and the chemical states associated with them."
A new way to study electrochemistry in action
With their model SOC, the Maryland-Sandia-Berkeley Lab team saw details never seen before in an operating fuel cell. Where an overall measurement gave only the fuel cell's total losses in potential energy, the APXPS measurements found the local potential losses associated with the interfaces of electrode and electrolyte, as well as with charge transport within the ceria electrode. The sum of the losses was equal to the cell's total loss, or inefficiency.
"The in situ XPS experiments at 750 C allowed us to pinpoint the electroactive regions, measure length scales of electron transport through mixed ionic-electronic conductors, and map out potential losses across the entire cell," Eichhorn says. "Others have suggested similar experiments in the past, but it was the remarkable facilities and scientific expertise at the ALS that facilitated these challenging measurements for the first time."
APXPS can provide this kind of fundamental information to solid oxide fuel cell designers, information not available using any other technique. New fuel cell designs are already taking advantage of this new way to study fuel cells in operation.
Original publication: Chunjuan Zhang et al.; "Measuring fundamental properties in operating solid oxide electrochemical cells by using in situ X-ray photoelectron spectroscopy"; Nature Materials, published online: 26 September 2010
These products might interest you
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!