Nanotubes: cellular membranes on supply
Osmotic forces play a role in nanotube formation in cells
When unfolding a tent for the first time, you may wonder how the huge tarpaulin fits into a bag the size of a football. Biologists wonder about something similar: when a cell divides, the surface area of the cell membrane grows. Moreover, when molecules are brought from one organelle to another inside the cell, membrane-enclosed transport vesicles are formed. So that membranes can be made available quickly, they are stored within the cells in the form of nanotubes, tubular membrane structures – similarly to a tarpaulin that has been folded together. Researchers at the Max Planck Institute of Colloids and Interfaces in Potsdam have now discovered a mechanism used by cells to generate stable membrane nanotubes.
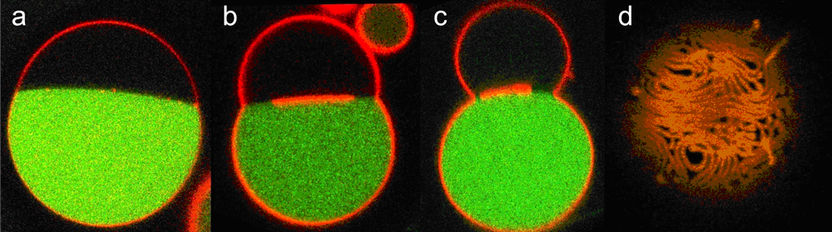
Nanotube formation in a vesicle containing two droplets (PEG - dark, and dextran - green). The membrane is labelled in red. After deflation of the vesicle, nanotubes form within the PEG-rich phase and accumulate at the interface between the two droplets. (a-c) Vertical cross sections of the vesicle; (d) top view of the nanotubes located at the interface.
Max Planck Institute of Colloids and Interfaces
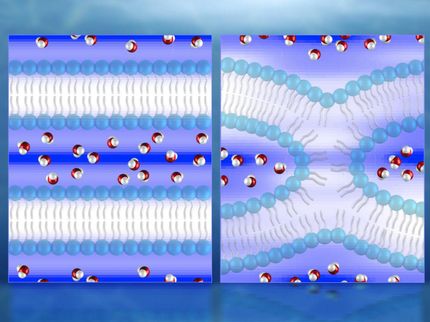
Tubular membrane structures can be found in many areas of a cell: in the Golgi apparatus, a type of sorting station in which transport vesicles are formed; in the mitochondria, the power plants of the cell; or in the endoplasmic reticulum, a type of duct network within cells. The tubes have a diameter ranging from a few nanometres (one millionth of a millimetre) to a few micrometres (one thousandth of a millimetre). The thinner the tubes, the greater the surface to volume ratio. They are therefore ideal for storing a lot of membrane in rather small spaces. Researchers believe that motor proteins can use energy to pull nanotubes from cellular membranes. “However, motor proteins are not always found in the areas of the cell where membrane nanotubes are formed,” says Rumiana Dimova, a researcher at the Max Planck Institute of Colloids and Interfaces and co-author of the study. For this reason, she believes that there must be another mechanism to generate stable nanotubes.
The Potsdam-based researchers may have now found the answer to the riddle. “The mechanism generates stable nanotubes without forces having to be exerted on the membrane. It therefore seems to work without the need for motor proteins,” says Dimova. Part of the mechanism is based on a phenomenon that is omnipresent in the world of membranes, the so-called osmosis. If certain molecules are present in a larger concentration outside the cell than inside the cell – i.e. they form a so-called hypertonic solution – then water will flow out of the cell and the cell will contract.
The researchers in Potsdam have reproduced such concentration differences using artificial vesicles the size of a cell, which contain a mixture of two polymers, namely polyethylene glycol (PEG) and dextran. “Biopolymers are found in a similarly high concentration in living cells,” says Dimova. “For this reason, we consider the vesicle to be a good model of a cell.” The researchers transferred the vesicle to a hypertonic solution, which caused the vesicle to release water and to shrink in volume.
However, what happened was completely different to a scenario in which, for instance, a beach ball is deflated and then simply collapses into a flat pancake. The outflow of the water caused the concentration of the dissolved polymers in the vesicle to rise. This, in turn, caused the two polymers to separate. As a result, two separate droplets of different sizes formed in the vesicle, much like the shape of a snowman with one large sphere (mainly containing PEG molecules) and one smaller sphere (predominantly containing dextran molecules).
Using a fluorescence microscope, the Potsdam-based researchers observed that membrane nanotubes formed in the PEG-rich area and accumulated at the interface between the two droplets. The scientists showed that about 15 % of the membrane surface had been stored in the tubes. The resolution of the microscope was not sufficient to be able to determine the diameter of the tubes. However, the researchers estimate it to be about 240 nanometres.
The researchers also have an explanatory model for the emergence and stability of the nanotubes. They found that solution flows of different densities are triggered when the polymers are separated. These exert forces on the membrane and thus contribute to the formation of the tubes.
The next question the scientists asked was what causes the membrane tubes to remain stable. A theoretical analysis of the observed membrane shapes revealed that stable tubes only emerge if the two sides of the membrane have an asymmetrical, molecular structure. This asymmetry is caused by the interaction between the membrane and the biopolymers. There is a high concentration of PEG molecules on one side, whereas on the other side there are no such molecules. Because the PEG interacts with the lipid molecules within the membrane, the membrane attempts to curve inwards. The formation of nanotubes accommodates this behaviour of the cellular membrane. The researchers observed that the nanotubes disappear again if the vesicle is allowed to inflate once again through osmosis.
“For natural cells, it is easy to generate asymmetry – similarly to what we have seen in our experiment,” says Dimova. The bio-physicist therefore believes that the newly discovered mechanism could be used in living cells to store the membrane surface. However, proof of this is still pending.
Original publication
Yanhong Li, Reinhard Lipowsky, Rumiana Dimova; "Membrane nanotubes induced by aqueous phase separation and stabilized by spontaneous curvature"; PNAS 2011.