Aqueous iron interacts as strong as solid iron
Advances spectroscopy research: HZB scientists come up with new method for examining the structure of metal ions-complexes in solution
HZB scientists have apply a new method -- "inverse Partial Fluorescence Yield" (iPFY) on micro-jet -- which will enable them to probe the electronic structure of liquids free of sample damages. The experiments are performed in vacuum conditions at the LiXEdrom experimental chamber, where a fluid stream of micrometer diameter is moving freely through vacuum and is continuously irradiated with X-ray radiation.
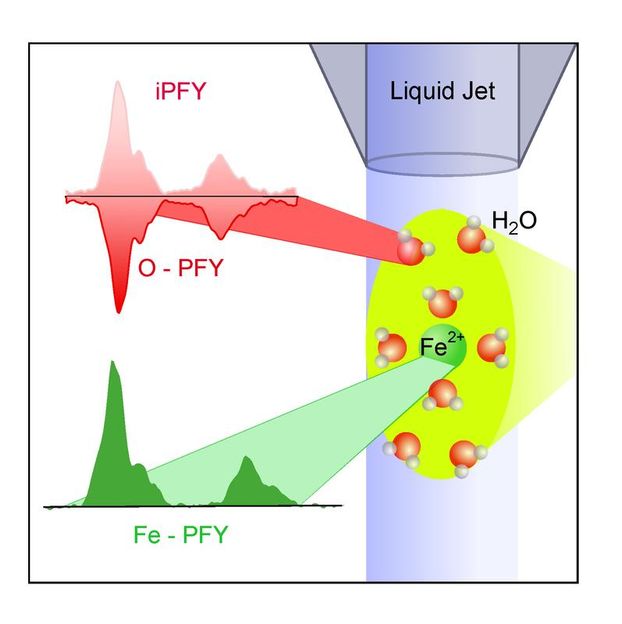
Metal ions in solution can be examined using soft X-ray radiation. In addition to metal ions, the free fluid stream in the vacuum also contains oxygen, which, following X-ray irradiation, begins to glow, ultimately affecting metal ion absorption. Researchers can now calculate the metal ions' absorptive strength and make inferences regarding the ions' electronic structures.
HZB
These kinds of experiments are important as they reveal the interaction strength of the X-rays with the liquids and therefore allow for the structural analysis of substances dissolved in solution. "The method will achieve its absolute apprehension when will be applied to metal ions that are part of chemical catalysts used for clean energy production and biocatalysts (protein enzymes) used in biochemical transformation inside the living cells – the team leader Prof. Aziz stated, which is the next milestone in our research progress. Previously, these types of experiments were so far only possible if the fluid was contained between two membranes, where radiation damages and membrane induced artifacts were a crucial issue. HZB's Young Investigator Group for Functional Materials in Solution headed by Prof. Dr. Emad Aziz has already applied the new method in iron ions dissolved in aqueous solution.
The researchers used X-ray radiation – generated by HZB's own electron storage ring BESSY II – to examine iron ions in aqueous solution. "We measured the absorption strength of the X-rays from our Fe 2+ and oxygen ions in the liquid micro-beam" explains Malte Gotz, who performed the experiments as part of his graduate research. "From here, we were able to draw conclusions regarding the electronic structure of the iron ions and further more to investigate the interaction of iron ions with the water solvent, " says Gotz.
The researchers used a new approach to measuring X-ray absorption of liquids. "Oxygen, which, along with iron ions, is also present in the solution, turns out to play a rather important role. If X-ray light is used to irradiate – and thereby the oxygen that is present in the water will absorb this radiation, and will end up emitting light for a brief period of time. You might compare it to the glow-in-the-dark of a clock," Gotz explains. If you now reduce the amount of incoming radiation by having a different material – in this case ionic iron absorbs it, it will directly reduce the amount of radiation emitted by the oxygen. "This in turn allows us to measure the absorption strength of ionic iron," says Gotz.
According to Emad Aziz, by definition, any measurement obtained at the free fluid stream is highly accurate. "A major advantage of our protocol is the fact that besides measuring only the signal from our fluid stream – without having to account for any artifacts induced by the surrounding container – we are also measuring a continuously fresh liquid sample," Aziz explains. In their studies the scientists found that iron ions suspended in the solution interact strongly with the solvent; a conclusion drawn by the strong 'Coster Kroenig decay process' observed in the liquid system, which were thus far observed only in solid iron. "We concluded that ions interact more strongly with water than was previously thought," says Aziz.
Our next step is to apply the new method to biological functional materials where the transition metals play key biological functions- such as oxygen-carrying iron in human blood. New and deep insights into these catalysts' structure and function are the challenge of our scientific research.