Computer model predicts red blood cell flow
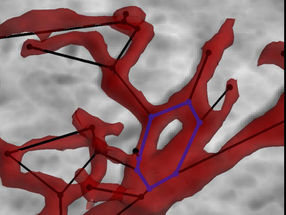
Adjacent to the walls of our arterioles, capillaries, and venules -- the blood vessels that make up our microcirculation -- there exists a peculiar thin layer of clear plasma, devoid of red blood cells. Although it is just a few millionths of a meter thick, that layer is vital. It controls, for example, the speed with which platelets can reach the site of a cut and start the clotting process.
"If you destroy this layer, your bleeding time can go way up, by 60 percent or more, which is a real issue in trauma," said Eric Shaqfeh, the Lester Levi Carter Professor and a professor of chemical engineering and mechanical engineering at Stanford University. Along with his colleagues, Shaqfeh has now created the first simplified computer model of the process that forms that layer -- a model that could help to improve the design of artificial platelets and medical treatments for trauma injuries and for blood disorders such as sickle cell anemia and malaria.
The thin plasma layer, known as the Fåhræus-Lindqvist layer, is created naturally when blood flows through small vessels. In the microcirculation, the layer forms because red blood cells tend to naturally deform and lift away from the vessel walls. "The reason they don't just continually move away from the wall and go far away is because, as they move away, then also collide with other red blood cells, which force them back," Shaqfeh explained. "So the Fåhræus-Lindqvist layer represents a balance between this lift force and collisional forces that exist in the blood."
Because the deformation of red blood cells is a key factor in the Fåhræus–Lindqvist layer, its properties are altered in diseases, such as sickle cell anemia, that affect the shape and rigidity of those cells. The new model, which is a scaled-down version of an earlier numerical model by Shaqfeh and colleagues that provided the first large-scale, quantitative explanation of the formation of the layer, can predict how blood cells with varying shapes, sizes, and properties -- including the crescent-shaped cells that are the hallmark of sickle cell anemia -- will influence blood flow.
The model can also help predict the outcome of -- and perfect -- treatments for trauma-related injuries. One common thing to do during treatment for trauma injuries is to inject saline, which among other things reduces the hematocrit, the blood fraction of red blood cells. With our model, Shaqfeh said, "we can predict how thick the Fåhræus-Lindqvist layer will be with a given hematocrit, and therefore how close the platelets will be to the periphery of the blood vessels -- and how quickly clotting will occur."
Original publication
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.



















![[Fe]-hydrogenase catalysis visualized using para-hydrogen-enhanced nuclear magnetic resonance spectroscopy](https://img.chemie.de/Portal/News/675fd46b9b54f_sBuG8s4sS.png?tr=w-712,h-534,cm-extract,x-0,y-16:n-xl)