Filming chemistry with a high speed x-ray camera
Chemistry happens all around us. A chemical reaction is a rearrangement of atoms in and between molecules, the breaking of old and the formation of new bonds. The glue that binds atoms in molecules an
Chemistry happens all around us. A chemical reaction is a rearrangement of atoms in and between molecules, the breaking of old and the formation of new bonds. The glue that binds atoms in molecules and creates the bonds between them is made out of valence electrons. Scientists of the Max-Born-Institut (MBI) in Berlin were able to show theoretically that the ultrafast x-ray camera is not only sensitive to inert core electrons but may also visualize the motion of chemically active valence electrons.
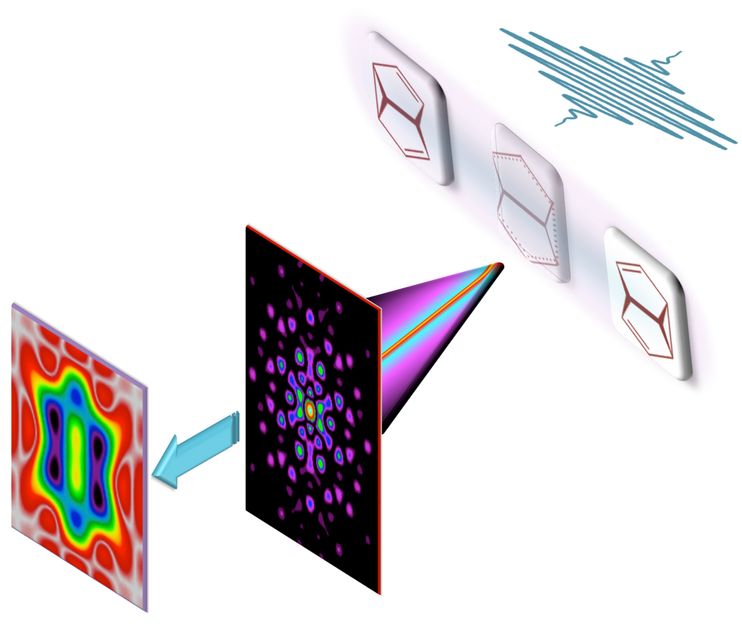
Filming bond making and bond breaking during a pericyclic reaction: We show theoretically that the ultrafast x-ray camera is not only sensitive to inert core electrons but may also visualize the motion of chemically active valence electrons.
MBI
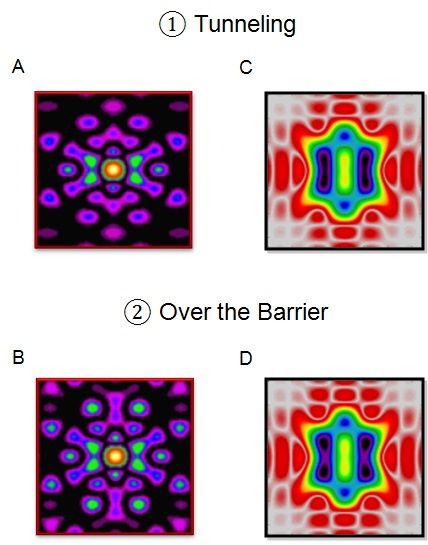
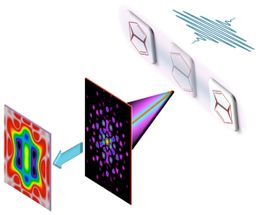
A combination of the standard analysis of the full x-ray scattering pattern (A, B) with an additional analysis of the part of the scattering pattern, which is limited to relatively small momentum transfer, one nearly effortlessly brings to the fore the motion of chemically active valence electrons during a pericyclic reaction (C, D). The breaking and making of chemical bonds along different reaction paths may thus be filmed and analyzed directly.
MBI
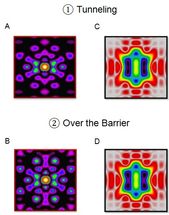
While the motion of valence electrons is at the very heart of chemical reactions, only a small fraction among them participates actively. The valence electron charge transferred between the atoms is often just a fraction of the charge of an electron. And those that do participate, do it very quickly: the duration of many very important chemical processes, such as first steps in vision and light harvesting, is measured in only tens to a hundred of femtoseconds. Making a movie of the chemically active electrons is therefore very challenging. First, one needs a camera with exquisite temporal and spatial resolution. Second, one needs a very sensitive camera. Indeed, one would really like to see not just how the atoms move, but also how the new bonds are formed as the old ones are broken - and this means filming the few active valence electrons in the sea of all electrons attached to the many atoms in the molecule.
An X-ray camera easily fits the first requirement. X-ray scattering has been indispensable in studying the structure of matter with atomic-scale spatial resolution since the discovery of x-rays. Thanks to enormous technological progress, it is now becoming possible to generate ultrashort flashes of x-rays, adding femtosecond temporal resolution to structural sensitivity. These flashes of x-rays promise to provide stroboscopic snapshots of chemical and biological processes in individual molecules.
However, fitting the second requirement - the sensitivity to active valence electrons - has never been the strength of an x-ray camera. X-ray scattering is always dominated by core and inert valence electrons. The small fraction of valence electrons actively participating in a chemical reaction is generally presumed lost in the scattering signal, seemingly placing ultrafast x-ray imaging of these electron densities out of the realm of possibility
The work suggests a way to resolve this challenge. In this work, we theoretically demonstrate a robust and effective method to extract the contributions of chemically active valence electrons from the total x-ray scattering by a single molecule - a critical step in the endeavor to film bond making and bond breaking as it happens, in space and time. The paper shows how, by combining the standard analysis of the full x-ray scattering pattern with an additional analysis of the part of the scattering pattern, which is limited to relatively small momentum transfer, one nearly effortlessly brings to the fore the motion of chemically active valence electrons.
The work not only showed how to film chemically active valence electrons with x-rays, it has also provided an experimental access to the long-standing problem: Are the new bonds made at the same time as old bonds are broken, or is there a time-delay between these two processes?
The x-ray camera confirms that the answer depends on whether the atoms have enough energy to climb over the energy barrier, which separates reactants from products, or if they have to resort to the quantum trick of tunneling through the energy barrier - the only option available when their energy is not sufficient to overcome it. In the first case the researchers confirm a time-delay between the breaking of old and the formation of new bonds. In the second case, they see no delay: the new bonds are built in concert with the destruction of the old ones.