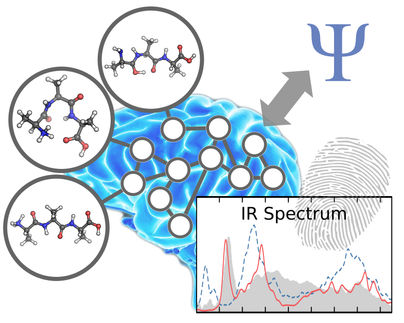
Protein reactions identified with subatomic resolution
Using subatomic resolution, researchers have gained insights into the dynamic modus operandi of two switch proteins which are responsible for the import of compounds into the nucleus and for cell growth. The team headed by Prof Dr Klaus Gerwert from the Department of Biophysics at the Ruhr-Universität Bochum, together with partners from Dortmund and Shanghai, combined different methods in order to gain a resolution of one-hundredth of the atomic diameter.
Switch proteins Ran and Ras control important processes in the cell
The switch proteins Ran and Ras control important processes such as the import of substances into the nucleus and cell growth. An impairment of their function may trigger severe diseases. A slowed down Ras protein, for example, causes intestinal cancer. Ran and Ras belong to the so-called GTPases. When the GTP molecule has bound to them, the proteins are switched on. If a phosphate group dissociates from the GTP molecule, the proteins are switched off. The team of Prof Dr Klaus Gerwert and PD Dr Carsten Kötting have been tracking that switch-off process in detail, together with colleagues from the Max Planck Institute of Molecular Physiology in Dortmund and the Partner Institute for Computational Biology in Shanghai.
Amino acids impair switch-off process
The Ran protein is switched off more slowly than the Ras protein. To date, researchers had assumed that this was linked to the position of a magnesium ion in the binding pocket for the GTP molecule. However, the current study has proved that the magnesium ion is located in exactly the same spot in Ran and Ras. Instead, the side chain of an amino acid in Ran prevents an attacking water molecule from assuming the optimal position for dissociating the phosphate group from GTP.
Resolution of one-hundredth of the atomic diameter achieved
For such detailed observations, a subatomic resolution is required. That is enabled through the combination of X-ray structure analysis, infrared spectroscopy and computer simulations. The X-ray structure analysis supplies graphic atomic models, which, however, are rigid. The infrared spectroscopy grants insights into dynamic processes with high temporal and spatial resolution; but it does not provide any graphic models. Using computer simulations, the data gathered via both these methods can be combined to generate high-resolution videos. The team from the Ruhr-Universität Bochum have been promoting the combination of computer simulations and IR spectroscopy.
Original publication
T. Rudack, S. Jenrich, S. Brucker, I. R. Vetter, K. Gerwert, C. Kötting; "Catalysis of GTP hydrolysis by small GTPases at atomic detail by integration of X-ray crystallography, experimental and theoretical IR spectroscopy"; Journal of Biological Chemistry; 2015
G. Schröter, D. Mann, C. Kötting, K. Gerwert; "Integration of Fourier Transform Infrared Spectroscopy, Fluorescence Spectroscopy, Steady-state Kinetics and Molecular Dynamics Simulations of Gαi1 distinguishes between the GTP hydrolysis and GDP release mechanism"; Journal of Biological Chemistry
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!