High-speed camera snaps bio-switch in action
X-ray experiment opens new route to study biochemical reactions
With a powerful X-ray camera, scientists have watched a genetic switch at work for the first time. The study led by Yun-Xing Wang from the National Cancer Institute of the U.S. reveals the ultrafast dynamics of a riboswitch, a gene regulator that can switch individual genes on and off. The innovative technique used for this investigation opens up a completely new avenue for studying numerous fundamental biochemical reactions.
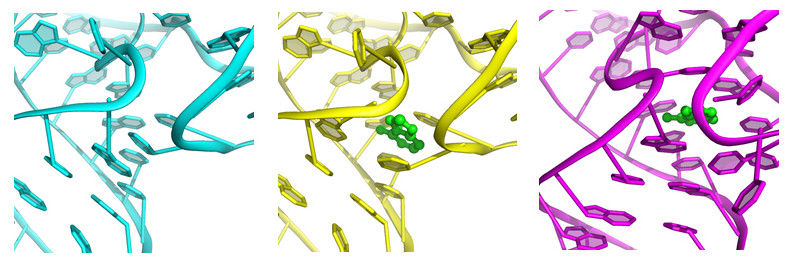
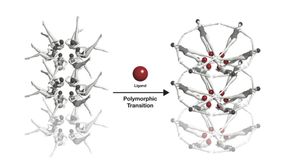
The riboswitch 'button' before, during and after coupling of the ligand (green), from left to right.
Yun-Xing Wang und Jason Stagno, National Cancer Institute
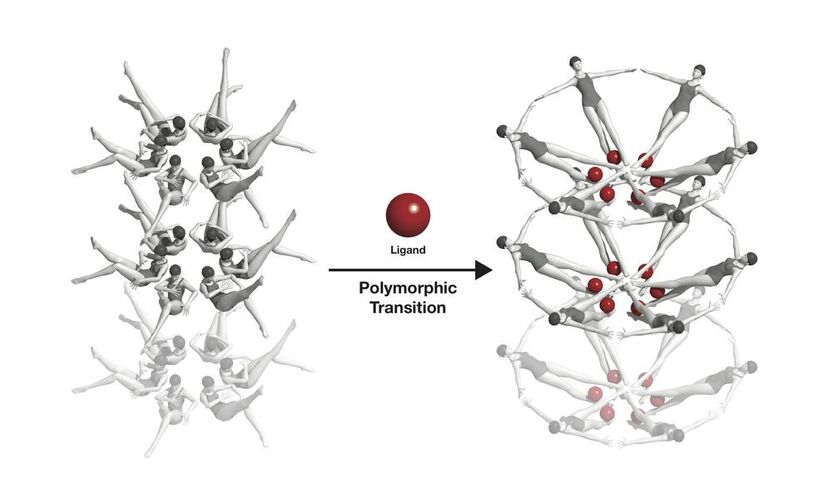
The riboswitche's conformational change, symbolised with swimmers: When a riboswitch meets a ligand (red), it changes its form.
Joseph Meyer, National Cancer Institute

“It's the proof that the structural changes occurring in biochemical reactions or interactions between molecules can be recorded in real time with the help of powerful X-ray lasers,” says co-author and DESY scientist Henry Chapman from the Center for Free-Electron Laser Science (CFEL), a cooperation of DESY, the University of Hamburg and the Max Planck Society.
The researchers studied a riboswitch from the bacterium Vibrio vulnificus, a close relative of the cholera germ. It can cause infections that are especially hard to treat and often fatal. The switch is activated by a signal molecule, known as a “ligand”, in this case adenine. Upon activation the riboswitch changes its form. As a result, the gene where the switch is located will no longer be read out and thus becomes deactivated. Riboswitches are present in bacteria and fungi, but not in mammals including humans. Targeting the genetic switches in bacteria might offer a powerful weapon in the fight against diseases.
To see how the switch is activated, the scientists crystallised its “button”, the part where the signal molecule binds, called an aptamer, since the molecular structure is formed from a sequence of nucleic acids rather than the usual amino acids of protein structures. The tiny aptamer crystals were injected into the ultra-bright beam of the X-ray laser LCLS at the SLAC National Accelerator Laboratory in California. Crystals scatter X-rays in characteristic ways, and from the resulting diffraction pattern the structure of the crystal can be calculated down to the atomic level, yielding the detailed structure of the aptamer in this case.
In a new device developed by Chapman's group, the aptamer crystals can be mixed with a solution of the signal molecule, adenine. The adenine diffuses into the crystal and “pushes the button” in the riboswitch, starting the biochemical interaction. The adenine soaked nanocrystals were injected into the beam of the X-ray laser, while a delay line allowed to freeze-frame the biochemical reaction a short time after it began.
This way, the scientists discovered an intermediate state of the riboswitch that has never been seen before and that likely only exists for milliseconds in a living organism. “Previous experiments at SLAC’s X-ray laser have studied biological reactions like photosynthesis that are triggered by light. But this is the first to observe one that is triggered by the chemical interaction of two biomolecules in real time and at the atomic scale,” says Wang. “This really demonstrates the unique capability that X-ray free-electron lasers offer that no current technology, or any other technology on the horizon, can do. It’s like you have a camera with a very fast shutter speed, so you can catch every move of the biomolecules in action.”
The study proves the concept of “mix-and-inject” crystallography to investigate biochemical reactions, explains Chapman, who is also a professor at the University of Hamburg and a member of the Hamburg Centre for Ultrafast Imaging (CUI).
With the conformational change of the aptamers, the entire crystal structure and symmetry changed. This requires very small crystals, as bigger crystals would simply fall apart due to the internal strain resulting from this rearrangement. Also, the crystals have to be small to allow a fast and even diffusion of the ligand solution. To analyse such tiny crystals, ultra-bright X-ray flashes are needed like those produced by the free-electron lasers LCLS or European XFEL that is currently being commissioned in the Hamburg and of which DESY is the main shareholder.
“Almost all proteins, RNAs, and DNAs interact with ligands or substrates and undergo certain conformation changes when they react. The ability to visualise these changes is critical to understanding how biomacromolecules perform their functions,” says Wang. “In the past, people studied these kinds of reactions indirectly or in very limited cases where the conformation changes were very small without cracking crystals. With our method, we are now able to directly visualise the structures and changes in real time in a much broader range of biochemical interactions and reactions.”
In addition to the National Cancer Institute and other National Institutes of Health, SLAC and DESY, the Arizona State University, the Johns Hopkins University, the Hauptmann-Woodward Medical Research Institute, the Structural Biology Center and the Argonne National Laboratory were involved in this research.