Iron outperforms gadolinium as MRI contrast agent
Rice University nanoscientists have demonstrated a method for loading iron inside nanoparticles to create MRI contrast agents that outperform gadolinium chelates, the mainstay contrast agent that is facing increased scrutiny due to potential safety concerns.
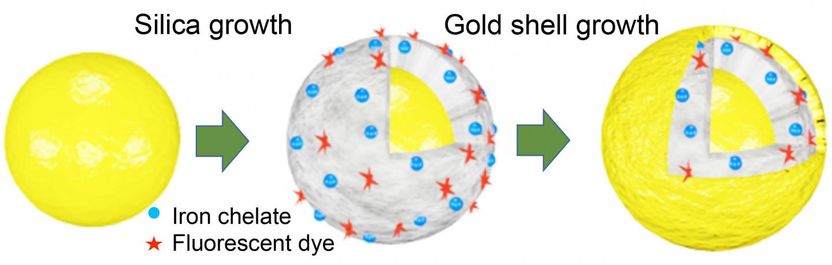
Scientists at Rice's Laboratory for Nanophotonics added iron chelates (blue) and fluorescent dye (red) to multi-layered gold nanomatryoshkas to create particles that can be used for disease therapy and diagnostics. The 'theranostic' nanoparticles have a core of gold (left) that is covered by silica containing the diagnostic iron and dye, which is covered by an outer shell of gold. The particles are about 20 times smaller than a red blood cell, and by varying the thickness of the layers, LANP scientists can tune the nanomatryoshkas to convert light into cancer-killing heat.
Luke Henderson/Rice University
"The possibility of eliminating gadolinium exposure and getting a two-fold improvement in T1 MRI contrast performance is going to intrigue radiologists," said Rice's Naomi Halas, the lead researcher on the project. "When they hear we've done this with iron I expect they will be very surprised."
Contrast agents are drugs that improve MRI images and make them easier for radiologists to interpret. Radiologists can "weight" the results of an MRI and make specific tissues appear either brighter or darker by varying the conditions of the test. Two weighting techniques -- T1 and T2 -- are used. While iron-based contrast agents are frequently employed for T2 scans, there are few clinically available alternatives to gadolinium for T1 tests.
"Iron chelates aren't new," Halas said. "It's widely believed they are wholly impractical for T1 contrast, but this study is a perfect illustration of how differently things can behave when you engineer at the nanoscale."
Halas and colleagues from Rice and the University of Texas MD Anderson Cancer Center describe their findings in a study. They created a modified version of nanomatryoshkas, concentric layered nanoparticles that draw their name from Russian nesting dolls.
Nanomatryoshkas and nanoshells, another layered nanoparticle Halas invented at Rice more than 20 years ago, are about 20 times smaller than a red blood cell and made up of layers of conductive metal and non-conductive silica. By varying the thickness of the layers, Halas' team tunes the particles to interact with specific wavelengths of light. For instance, both nanoshells and nanomatryoshkas can convert otherwise harmless near-infrared light to heat. This localized, intense heating has been used to destroy cancer in several trials of nanoshells, including an ongoing trial for the treatment of prostate cancer.
The new study is the latest chapter in Halas' efforts to create light-activated nanoparticles with a combination of therapeutic and diagnostic features. These "theranostic" particles could allow clinicians to diagnose and treat cancer in the same office or hospital visit.
Luke Henderson, a Rice graduate student, said, "If clinicians could visualize the particles through some sort of imaging, therapy could be faster and more effective. For example, imagine a scenario where a scan is performed to verify the size and placement of the tumor, heat is then generated to treat the tumor and another scan follows to verify that the entire tumor was destroyed."
When Henderson, a chemist, joined Halas' Laboratory for Nanophotonics in 2016, Halas' team had already shown it could add fluorescent dyes to nanomatryoshkas to make them visible in diagnostic scans. Work was also underway on a study published in 2017 that showed gadolinium chelates could be embedded in the silica layer for MRI contrast.
MRI scanners image the body's interior by briefly aligning the nuclei of hydrogen atoms and measuring how long it takes the nuclei to "relax" to their resting state. Relaxation properties vary by tissue, and by repeatedly aligning nuclei and measuring relaxation times, an MRI scanner builds a detailed image of the body's organs, tissues and structures. Contrast agents improve scan resolution by increasing the relaxation rate of particles.
Gadolinium chelates revolutionized MRI testing when they were introduced in the late 1980s and have been used more than 400 million times. Though gadolinium is a toxic metal, the chelating process covers each gadolinium ion with an organic wrap that reduces exposure and allows the drug to pass from the body via urination within a few hours
In 2013, Japanese scientists made the surprising discovery that gadolinium from contrast agents had accumulated in the brains of some patients, and subsequent studies found similar deposits in bones and other organs. While no adverse health effects have been associated with gadolinium-based MRI contrast agents, the FDA required drug makers to add warnings to the medication guides for eight widely used gadolinium-based contrast agents in December 2017.
"In the earlier work with gadolinium, we noticed that the nanomatryoshka design enhanced the relaxivities of the embedded gadolinium chelates," Henderson said. "At the same time, we were hearing more calls from the medical community for alternatives to gadolinium, and we decided to try iron chelates and see if we got the same sort of enhancement."
The results surprised everyone. Not only was Henderson able to boost the relaxivities for iron, he was able to load about four times more iron into each nanomatryoshkas. That allowed the iron-laden nanomatryoshkas to perform twice as well as clinically available gadolinium chelates.
Henderson also found a generic way to change the type of metal that was loaded. By adding unloaded chelate molecules to the silica first, he found he could load metal by soaking the particles in a bath of metal salts. By changing the metals in the bath, he found he could easily load different paramagnetic ions, including manganese, into the nanomatryoshkas.
After the metal ions were loaded into the silica, the final layer of the nanomatryoshka, the outer gold shell, was added. The shell, which is vital for plasmonics, also serves as barrier to prevent ion leeching. Henderson said the gold barrier also had a secondary benefit for the fluorescent dyes he added for dual-mode diagnostics.
"All fluorescent dyes are subject to photo bleaching, which means they fade over time and eventually won't give off a measureable signal," Henderson said. "Even if you freeze them, which slows down bleaching, they typically don't last more than a couple of weeks. I was looking at an old sample of nanomatryoshkas that had been in the fridge for months, and I found they were still fluorescing quite well. When we looked more closely at this we found the dyes were about 23 times more stable when they were inside the nanomatryoshkas."
Original publication
Luke Henderson, Oara Neumann, Caterina Kaffes, Runmin Zhang, Valeria Marangoni, Murali K. Ravoori, Vikas Kundra, James Bankson, Peter Nordlander, and Naomi J. Halas; "Routes to Potentially Safer T1 Magnetic Resonance Imaging Contrast in a Compact Plasmonic Nanoparticle with Enhanced Fluorescence"; ACS Nano; 2018