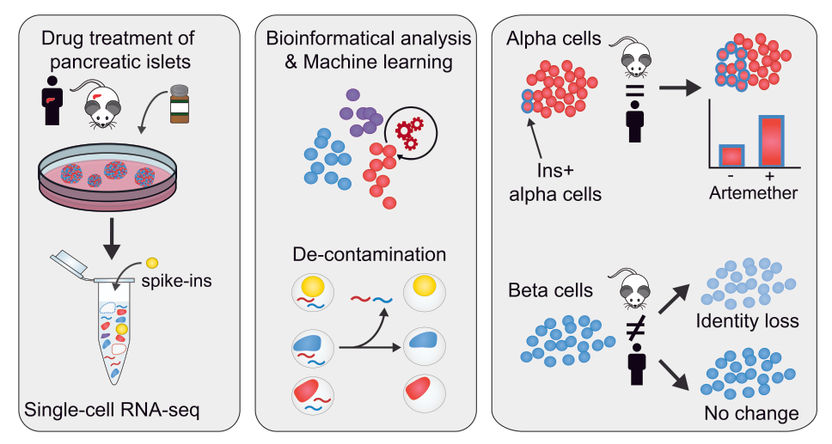
bioMerieux to Develop New Highly-Specific, Non-Invasive Test for Prostate Cancer, Reducing Unnecessary Biopsies
bioMérieux has signed a license and development agreement with the German biotechnology company, ProteoSys, for Annexin 3, which will be used to develop a urine-based, confirmatory diagnostic test for prostate cancer. After a research phase, the new test should be developed on the VIDAS® platform.
Annexin 3, also known as ANXA 3, was discovered by ProteoSys, a German biotechnology company based in Mainz, which specializes in the fields of cell biology and proteomics. Studies have shown that ANXA 3 quantification in urine is a novel, non-invasive test with high specificity for prostate cancer. Today, when the levels of PSA (Prostate Specific Antigen) are in the uninformative “grey zone”, a biopsy is used to provide definitive diagnosis. The ANXA 3 test would be used to provide better identification of patients with a high probability of prostate cancer, thereby reducing the number of unnecessary biopsies.
The first phase of research is beginning at bioMérieux, which will be followed by the development of a diagnostic test for the VIDAS platform. While the confirmatory diagnostic application on VIDAS will be the initial focus, bioMérieux is also considering the development of treatment decision and prognostic applications for ANXA 3, as described in a leading publication2. The financial details of the deal are not disclosed.
Original Publications: Schostak et al.; "Annexin A3 in urine – a highly specific non invasive marker in prostate cancer early detection."; J. Urol. 2008.
Köllermann J, Schlomm T, Bang H, Schwall GP, von Eichel-Streiber C, Simon R, Schostak M, Huland H, Berg W, Sauter G, Klocker H, Schrattenholz A; "Expression and Prognostic Relevance of Annexin A3 in Prostate Cancer."; Eur Urol. 2008.
Most read news
Topics
Organizations
Other news from the department business & finance

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents