Copper(I) interlocks rings and rods
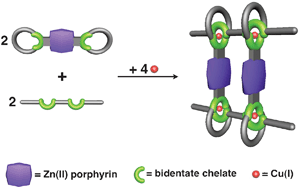
Scientists in France have synthesised highly functional [4]pseudorotaxanes utilising the gathering and threading effect of copper(I). Jean-Pierre Sauvage, Jean-Paul Collin, Valérie Heitz and colleagues from the University of Strasbourg, in France, have prepared a highly functional interlocking system consisting of four independent organic fragments (two bis-macrcycles and two rod-like compounds which are threaded through the rings) and four copper(I) metal centres. This self-assembly process relies on the formation of coordination chemistry bonds between the copper centres and nitrogen that form easily and quantitatively. Of particular significance is the high functionality of the system which is due to two zinc complexed porphyrins incorporated as lateral plates, and the rods and rings containing chelating groups. Originally the motivation to produce assemblies of interlocking rings (catenanes) or rings threaded by string-like fragments (rotaxanes) was the synthetic challenge. However these structures possess new properties that are useful in the fields of photochemistry, photochemical sensors, electron transfer, host-guest chemistry and molecular machines. ‘The construction principle in these systems, based on coordination chemistry can be generalised to form more complex edifices towards the fabrication of molecular devices,’ says Sauvage. Original article: Sauvage et. al.; "Quantitative formation of [4]pseudorotaxanes from two rods and two bis-macrocycles incorporating porphyrinic plates between the rings"; Chem. Commun. 2009
Most read news
Topics
Organizations
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.