Phosphorylated peptide detection
Itaru Hamachi and colleagues from Kyoto University have created a chemosensor that shows a high binding selectivity towards (i, i+1) bisphosphorylated peptide, displaying a dual-emission fluorescence change.
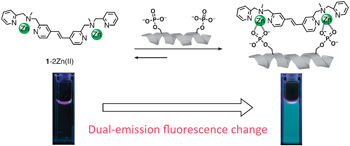
Fluorescent Chemosensor for phosphorylated peptide.
'Development of artificial chemosensors that can detect phosphorylated proteins or peptides is useful to analyze their functions and the activities of relevant protein kinase and phosphatase,' Hamachi explains. 'This helps our understanding of cell signaling and the development of therapeutics and medical diagnosis for the related disease.'
'Sequence- or site-specific detection of a certain protein phosphorylation with an artificial chemosensor still remains a challenging task' says Hamachi. 'We have developed a new fluorescent chemosensor on the basis of a unique molecular design; the two phosphate recognition sites are directly conjugated with a rigid fluorophore.'
Hamachi explains that the high binding selectivity of their chemosensor towards (i, i+1) bis-phosphorylated peptide, and the clear dual-emission change on formation of the binding complex, cannot be readily achieved with previously reported chemosensors. 'We anticipate that the present molecular design would be generally applicable for sequence selective detection of various types of (i, i+n) bis-phosphorylated peptides, by adjustable elongation of the olefine spacer unit between the two phosphate recognition sites.'
Original article: Ishida et al.; Chem. Commun. 2009
Topics
Organizations
Other news from the department science
These products might interest you

Kjel- / Dist Line by Büchi
Kjel- and Dist Line - steam distillation and Kjeldahl applications
Maximum accuracy and performance for your steam distillation and Kjeldahl applications

AZURA Purifier + LH 2.1 by KNAUER
Preparative Liquid Chromatography - New platform for more throughput
Save time and improve reproducibility during purification

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.