Iowa State, Ames Lab chemists discover how antiviral drugs bind to and block flu virus
Antiviral drugs block influenza A viruses from reproducing and spreading by attaching to a site within a proton channel necessary for the virus to infect healthy cells, according to a research project led by Iowa State University's Mei Hong and published in Nature.
Hong, Iowa State's John D. Corbett Professor of Chemistry and an associate scientist for the U.S. Department of Energy's Ames Laboratory, said the findings clarify previous, conflicting studies and should pave the way to development of new antiviral drugs against influenza viruses, including pandemic H1N1.
Two papers published by Nature in 2008 came to different conclusions about where the antiviral drug amantadine binds to a flu virus and stops it from infecting a healthy cell. A paper based on X-ray studies concluded the drug attached to the lumen of the proton channel, the area inside the channel, and stopped the virus by blocking the channel. Another paper based on solution nuclear magnetic resonance (NMR) technology concluded the drug attached to the surface of the virus protein near the proton channel and stopped the virus by indirectly changing the channel structure.
Hong's research concluded that when amantadine is present at the pharmacologically relevant amount of one molecule per channel, it attaches to the lumen inside the proton channel. But the paper also reports that when there are high concentrations of amantadine in the membrane, the drug will also attach to a second site on the surface of the virus protein near the channel.
"Our study using solid-state NMR technology unequivocally shows that the true binding site is in the channel lumen, while the surface-binding site is occupied only by excess drug," Hong said. "The previous solution NMR study used 200-fold excess drug, which explains their observation of the surface-binding site. The resolution of this controversy means that medical chemists can now try to design new drugs to target the true binding site of the channel."
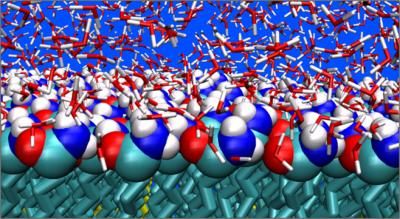
Here's how a flu virus uses its proton channel and how amantadine blocks that channel: The virus begins an infection by attaching itself to a healthy cell. The healthy cell surrounds the flu virus and takes it inside the cell through a process called endocytosis. Once inside the cell, the virus uses a protein called M2 to open a channel to the healthy cell. Protons from the healthy cell flow through the channel into the virus and raise its acidity. That triggers the release of the virus' genetic material into the healthy cell. The virus hijacks the healthy cell's resources and uses them to reproduce and spread. When amantadine binds to and blocks the M2 proton channel, the process doesn't work and a virus can't infect a cell and spread.
Hong and the research team developed powerful techniques to study the proton channel using solid-state NMR spectroscopy, the technology behind medical magnetic resonance imaging. The techniques provided the researchers with a detailed look at the antiviral drug within the proton channel, showed them the structure of the protein at the drug-binding site and allowed them to make accurate measurements of the distances between the drug and the protein.
The researchers also found that amantadine spins when it binds to the inside of the proton channel. That means it doesn't fill the channel. And Hong said that leaves room for development of other drugs that do a better job blocking the channel, stopping the flu and evading development of drug resistance.
Most read news
Topics
Organizations
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.